
What is the difference between molar mass and atomic mass?
Answer
493.2k+ views
Hint: To solve this question, we should know about the concept of atoms. Molar mass is the mass of the one mole of the compound whereas atomic mass is the mass of the individual unit of the compound. Basically, molar mass is the mass of an average of many elements of the compound and atomic mass is the mass of the atom.
Complete answer:
For example, let us take hydrogen chloride $(HCl)$ .
The atomic mass of $HCl$ can be calculating by using atomic masses of the individual elements found on the periodic table:

This is equivalent to the molar mass of $HCl$ , that is:
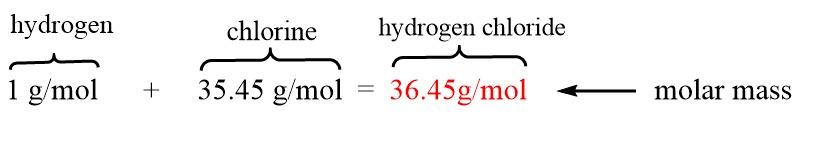
So, the atomic mass of the compound $HCl$ is $36.45\;amu$ , and the molar mass is $36.45gmo{l^{ - 1}}$ .
Note:
We should ensure not to be confused between the two terms, atomic mass and atomic weight. Atomic mass is the mass of an atom whereas atomic weight is the weight of the average of the naturally occurring isotopes.
Complete answer:
| Molar mass | Atomic mass |
| The molar mass of the compound is defined as the total mass of atoms in grams present in a mole of a molecule. | On the other hand, atomic mass is termed as the mass of the atom. |
| In other words, it is the mass of one mole of the compound or Avogadro number of particles, i.e. $6.022 \times {10^{23}}$ particles expressed in grams. | It can also be defined as the number of protons and the number of neutrons. |
| The molar mass is the average of many samples of the compound which often vary in mass because of the presence of isotopes. The S.I. The unit of molar mass is $Kgmo{l^{ - 1}}$ . | The S.I. The unit of atomic mass is amu , which is defined as $\dfrac{1}{{12}}$ of the mass of a single $carbon - 12$ atom. |
For example, let us take hydrogen chloride $(HCl)$ .
The atomic mass of $HCl$ can be calculating by using atomic masses of the individual elements found on the periodic table:

This is equivalent to the molar mass of $HCl$ , that is:
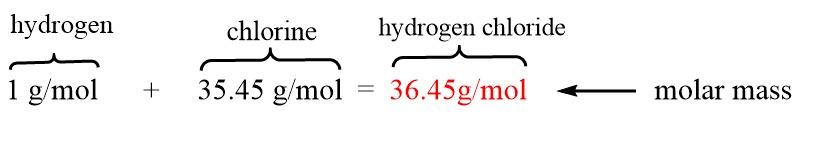
So, the atomic mass of the compound $HCl$ is $36.45\;amu$ , and the molar mass is $36.45gmo{l^{ - 1}}$ .
Note:
We should ensure not to be confused between the two terms, atomic mass and atomic weight. Atomic mass is the mass of an atom whereas atomic weight is the weight of the average of the naturally occurring isotopes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




