
What is the difference between a staggered, eclipsed, gauche, and anti-conformation?
Answer
538.2k+ views
Hint: The end-to-end overlap of orbitals in sigma bonds make them free to rotate in organic molecules. Due to this rotation of the sigma bond, different arrangements of a molecule are possible that are known as conformations. The main difference between types of conformations is the dihedral angle, potential energy, and spatial arrangement of atoms around a single bond.
Complete answer:
The bond formation in alkane takes place by the end-to-end overlapping of orbitals that results in the formation of a sigma bond. Due to such bonding, the atoms can freely rotate around the C-C single bond. Such rotation results in different spatial arrangements of alkanes which can be interconverted by rotation around the C-C bond and are known as conformations or conformational isomers.
To understand the different types of conformations, let us consider an example of butane. There are a total of 3 rotating carbon bonds but we will consider the rotation between the C2-C3 bond. The conformations are represented using Newman projections, in which out of the two rotating carbons, the closer one is shown as a dot while the rear one is shown as a circle.
There can be an infinite number of conformations by rotation around the C-C bond that is not completely free due to repulsion between electron clouds of C-H bonds. This repulsive interaction is called torsional strain.
Each conformation represents a state of different potential energy. When repulsive forces are strong, conformations vary greatly in energy, and the molecule will occupy a steady state and experience a change to another steady-state upon absorbing enough energy.
Below are the four main types of conformations that are known for the butane molecule. The first one is known as eclipsed conformation as the two methyl groups are one behind another. This is maximum energy conformation and thus most unstable due to steric repulsion between two bulky methyl groups.
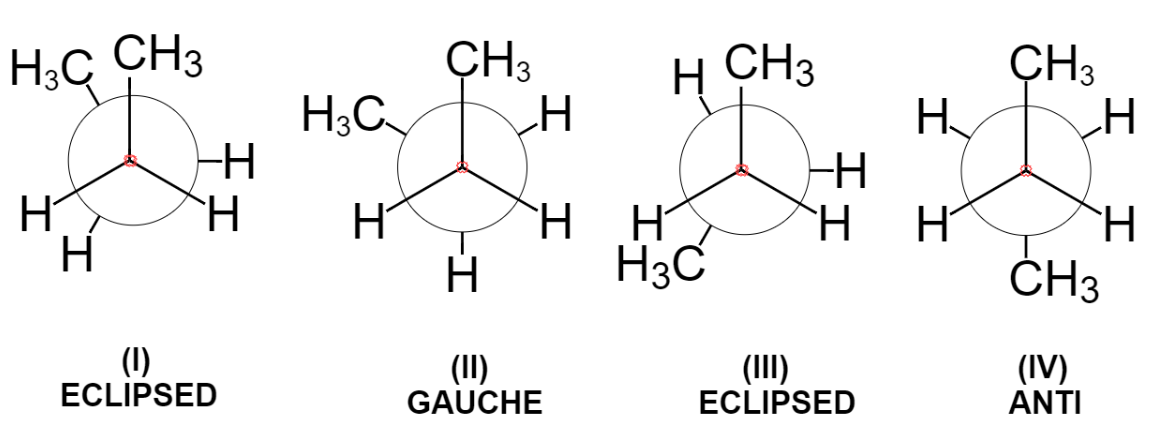
Now, turning the front carbon by 60 degrees clockwise, we get the gauche conformation in which two methyl groups are somewhat far apart still the repulsion between two exists.
Further rotation of 60 degrees results again in the formation of eclipsed conformation in which methyl groups are behind the hydrogen atoms. Due to steric repulsion between methyl and hydrogen, this conformation is less stable than gauche but more stable than eclipsed conformation (I).
Again, rotation of 60 degrees gives the anti-conformation where the two bulky groups are situated opposite to each other and thus it is the most stable conformation due to minimum steric repulsion.
Hence, these four conformations differ mainly in energy and spatial arrangement.
Note:
At room temperature, butane is expected to be present in the lowest energy anti confirmation at any time, however, the energy barrier is not high enough to stop the continuous rotation of conformers except at very low temperatures.
Complete answer:
The bond formation in alkane takes place by the end-to-end overlapping of orbitals that results in the formation of a sigma bond. Due to such bonding, the atoms can freely rotate around the C-C single bond. Such rotation results in different spatial arrangements of alkanes which can be interconverted by rotation around the C-C bond and are known as conformations or conformational isomers.
To understand the different types of conformations, let us consider an example of butane. There are a total of 3 rotating carbon bonds but we will consider the rotation between the C2-C3 bond. The conformations are represented using Newman projections, in which out of the two rotating carbons, the closer one is shown as a dot while the rear one is shown as a circle.
There can be an infinite number of conformations by rotation around the C-C bond that is not completely free due to repulsion between electron clouds of C-H bonds. This repulsive interaction is called torsional strain.
Each conformation represents a state of different potential energy. When repulsive forces are strong, conformations vary greatly in energy, and the molecule will occupy a steady state and experience a change to another steady-state upon absorbing enough energy.
Below are the four main types of conformations that are known for the butane molecule. The first one is known as eclipsed conformation as the two methyl groups are one behind another. This is maximum energy conformation and thus most unstable due to steric repulsion between two bulky methyl groups.

Now, turning the front carbon by 60 degrees clockwise, we get the gauche conformation in which two methyl groups are somewhat far apart still the repulsion between two exists.
Further rotation of 60 degrees results again in the formation of eclipsed conformation in which methyl groups are behind the hydrogen atoms. Due to steric repulsion between methyl and hydrogen, this conformation is less stable than gauche but more stable than eclipsed conformation (I).
Again, rotation of 60 degrees gives the anti-conformation where the two bulky groups are situated opposite to each other and thus it is the most stable conformation due to minimum steric repulsion.
Hence, these four conformations differ mainly in energy and spatial arrangement.
Note:
At room temperature, butane is expected to be present in the lowest energy anti confirmation at any time, however, the energy barrier is not high enough to stop the continuous rotation of conformers except at very low temperatures.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




