
Diazo coupling is useful to prepare some:
(a)- Pesticides
(b)- Dyes
(c)- Proteins
(d)- Vitamins
Answer
590.4k+ views
Hint: The product formed by the diazo coupling reaction is a colored compound and is used to color other compounds. Methyl orange is an example of a compound formed by the diazo coupling reaction.
Complete answer:
Arenediazonium salts react with highly reactive (i.e., electron-rich) aromatic compounds such as phenols and amines to form highly brightly colored azo compounds $Ar-N=N-Ar$. This reaction is called a coupling reaction and is extensively used in the azo dye industry. Whereas coupling with phenols occurs in the basic medium (pH 9-10) that of amines occurs in the faintly acidic medium at 273-278 K.
The color of azo compounds is due to extended conjugation involving the double bonds of both the arene (benzene) rings through a $-N=N-$ double bond.
A coupling reaction is an example of an electrophilic substitution reaction in which the diazonium cation with the positive charge on the terminal nitrogen acts as the electrophile while the electron-rich compound such as phenols and amines acts as the nucleophile.
When benzene diazonium chloride reacts with phenol it forms p-Hydroxyazobenzene which is an orange dye.
The reaction is given below:
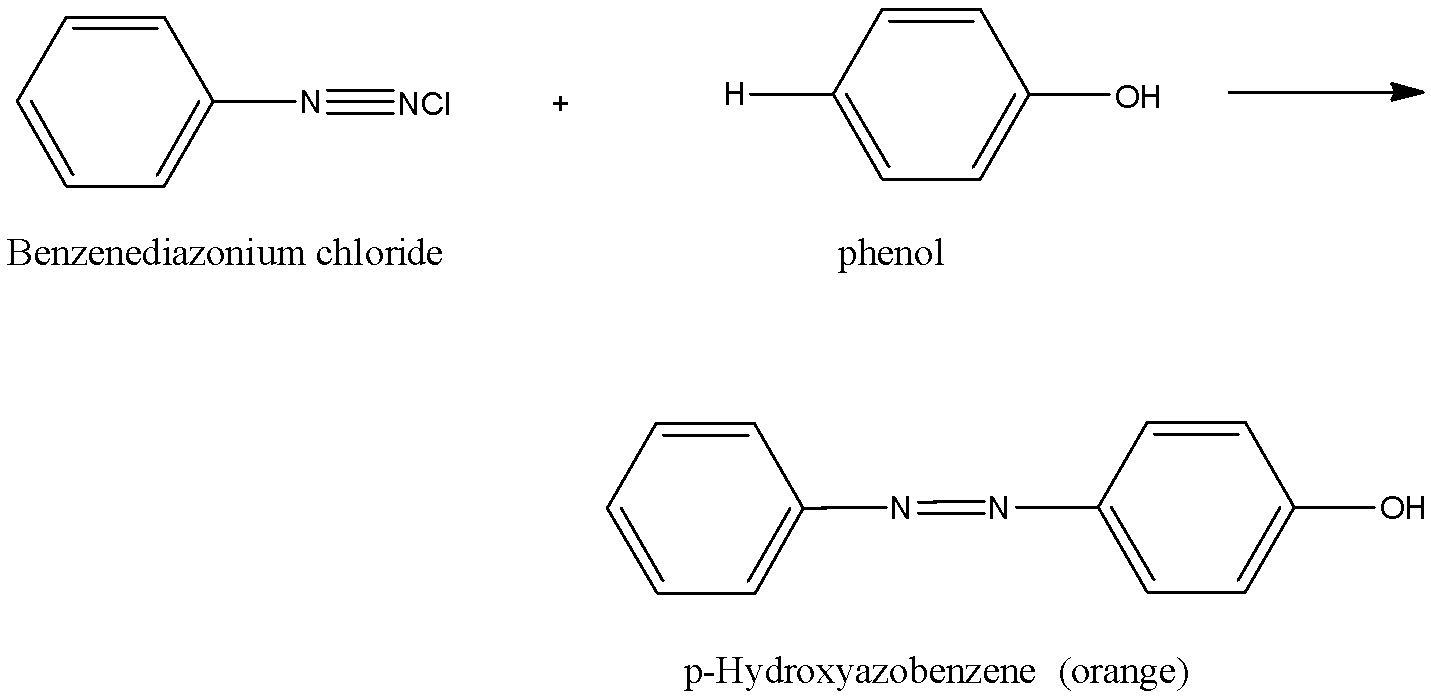
When benzene diazonium chloride reacts with aniline it forms p-Aminoazobenzene which is a yellow dye.
The reaction is given below:
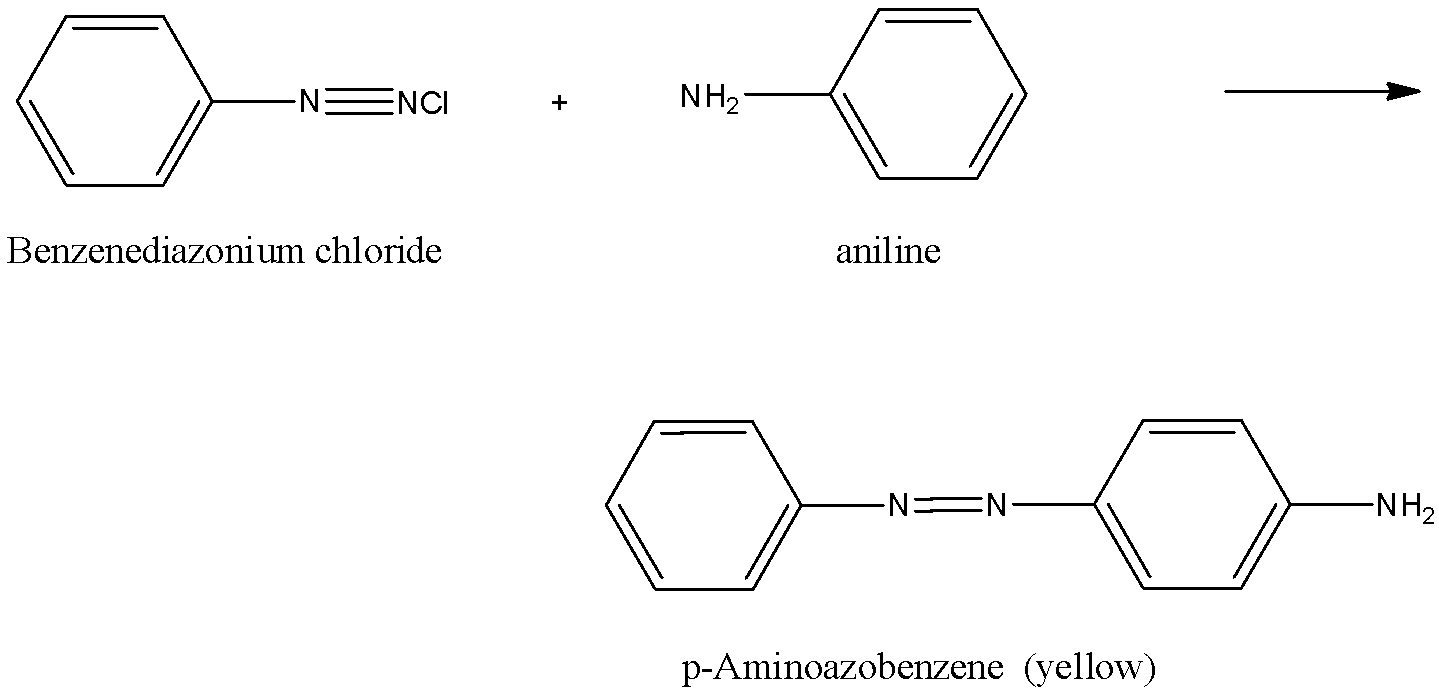
Hence, the correct answer is an option (b)- Dyes.
Note:
The well-known indicator methyl orange which is widely used in acid-base titrations is obtained by coupling the diazonium salt of sulfanilic acid with N, N-dimethylaniline.
The reaction is:
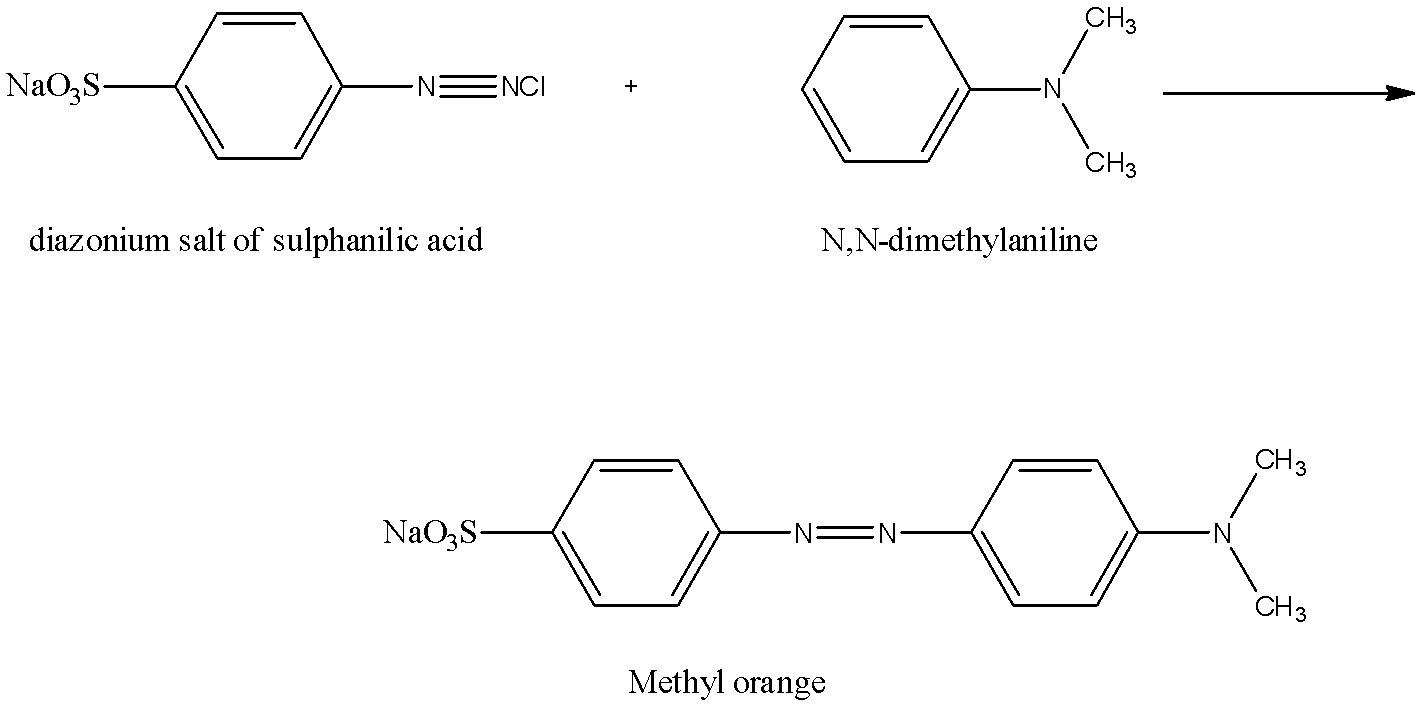
Complete answer:
Arenediazonium salts react with highly reactive (i.e., electron-rich) aromatic compounds such as phenols and amines to form highly brightly colored azo compounds $Ar-N=N-Ar$. This reaction is called a coupling reaction and is extensively used in the azo dye industry. Whereas coupling with phenols occurs in the basic medium (pH 9-10) that of amines occurs in the faintly acidic medium at 273-278 K.
The color of azo compounds is due to extended conjugation involving the double bonds of both the arene (benzene) rings through a $-N=N-$ double bond.
A coupling reaction is an example of an electrophilic substitution reaction in which the diazonium cation with the positive charge on the terminal nitrogen acts as the electrophile while the electron-rich compound such as phenols and amines acts as the nucleophile.
When benzene diazonium chloride reacts with phenol it forms p-Hydroxyazobenzene which is an orange dye.
The reaction is given below:

When benzene diazonium chloride reacts with aniline it forms p-Aminoazobenzene which is a yellow dye.
The reaction is given below:

Hence, the correct answer is an option (b)- Dyes.
Note:
The well-known indicator methyl orange which is widely used in acid-base titrations is obtained by coupling the diazonium salt of sulfanilic acid with N, N-dimethylaniline.
The reaction is:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




