
What is diagonal relationship? Give one example.
Answer
606.6k+ views
Hint: A relationship exists between certain diagonally adjacent elements of the s-block due to which they have similar properties. Such relationships exist because moving rightward and descending the periodic table have opposite effects.
Complete answer:
Some pairs of diagonally adjacent elements of s-block in the second and third periods of the periodic table exhibit similar properties. Most of the properties follow opposite trends when moving along a period and down a group. So, the changes more or less cancel each other out, and elements with similar properties can be found. On moving along a period, the elements become more covalent, more electronegative and less basic. On the other hand, on moving down a group, the elements become more ionic, less electronegative and more basic. So, the atomic radius, electronegativity, properties of compounds, etc. of the diagonal elements are similar.
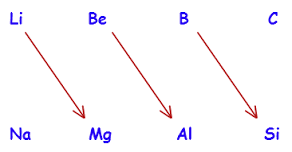
The properties of the elements of first-group second-period are often similar to the properties of the elements of second-group third-period. Thus, the properties of Li are similar to Mg, the properties of Be are similar to that of Al and B and Si also have the same properties.
For the Li-Mg pair:
- Lithium reacts slowly with water as compared to other Group 1 elements, alike Mg.
- The hydroxides of both the elements are weak bases and decompose on heating.
- Li is the only element of Group 1 which forms a stable nitride . Mg and other Group 2 also form nitrides.
- Li and Mg both form covalent organometallic compounds while the rest of Group 1 and Group 2 compounds analogues are ionic and very reactive.
- Metals of Group 1, excluding Li form peroxides and superoxides when reacted with oxygen under standard conditions. Li, on the other hand, forms normal oxides like Mg.
- Heating properties of carbonates of Li and Mg are similar. [Both are unstable and produce corresponding oxides and carbon dioxide upon heating].
Note: Some might consider diagonal relationship past B and Si too which is wrong. Practically, after B-Si pair the diagonal relationship becomes less dominant.
Complete answer:
Some pairs of diagonally adjacent elements of s-block in the second and third periods of the periodic table exhibit similar properties. Most of the properties follow opposite trends when moving along a period and down a group. So, the changes more or less cancel each other out, and elements with similar properties can be found. On moving along a period, the elements become more covalent, more electronegative and less basic. On the other hand, on moving down a group, the elements become more ionic, less electronegative and more basic. So, the atomic radius, electronegativity, properties of compounds, etc. of the diagonal elements are similar.

The properties of the elements of first-group second-period are often similar to the properties of the elements of second-group third-period. Thus, the properties of Li are similar to Mg, the properties of Be are similar to that of Al and B and Si also have the same properties.
For the Li-Mg pair:
- Lithium reacts slowly with water as compared to other Group 1 elements, alike Mg.
- The hydroxides of both the elements are weak bases and decompose on heating.
- Li is the only element of Group 1 which forms a stable nitride . Mg and other Group 2 also form nitrides.
- Li and Mg both form covalent organometallic compounds while the rest of Group 1 and Group 2 compounds analogues are ionic and very reactive.
- Metals of Group 1, excluding Li form peroxides and superoxides when reacted with oxygen under standard conditions. Li, on the other hand, forms normal oxides like Mg.
- Heating properties of carbonates of Li and Mg are similar. [Both are unstable and produce corresponding oxides and carbon dioxide upon heating].
Note: Some might consider diagonal relationship past B and Si too which is wrong. Practically, after B-Si pair the diagonal relationship becomes less dominant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




