
$D-glucose\xrightarrow{O{{H}^{-}}}A+B$
A and B are:
A. D-mannose and D-mannitol
B. D-mannose and D-Fructose
C. D-allose and D-Altrose
C. D-Glucose and D-idose
Answer
590.4k+ views
Hint: It is a reaction which is basically acid or base catalysed transformation of an aldose into the ketose or vice-versa. In this a reaction intermediate was formed called as enediol.
Complete step by step answer:
- The reaction given here is called the Lobry de Bruyn-van Ekenstein reaction. We will write the reaction as: $D-glu\cos e\xrightarrow{O{{H}^{-}}}D-mannose+D-Fructose$
- Structurally, we can represent it as:
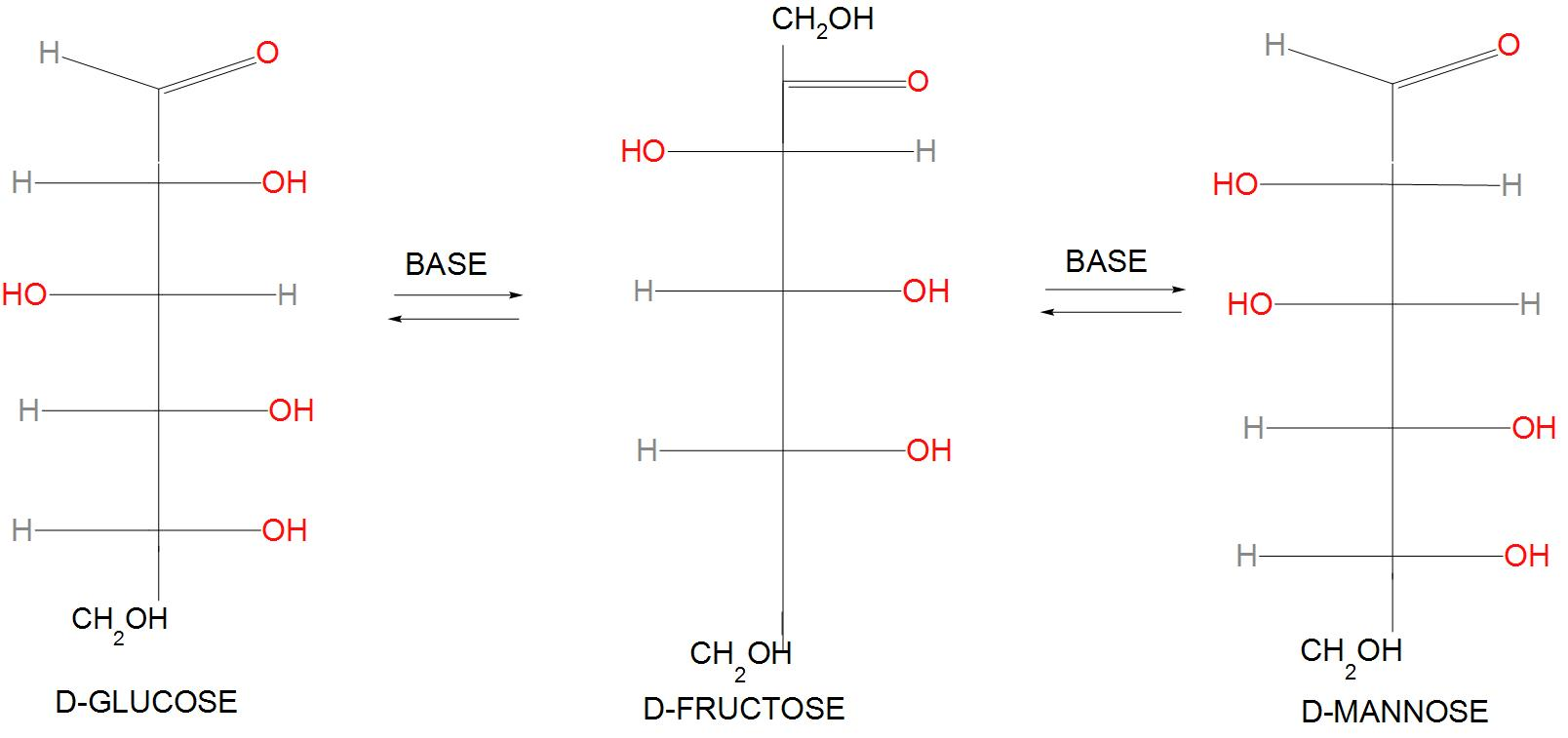
- Firstly, we are being given with D-Glucose. The structure of D-Glucose is:
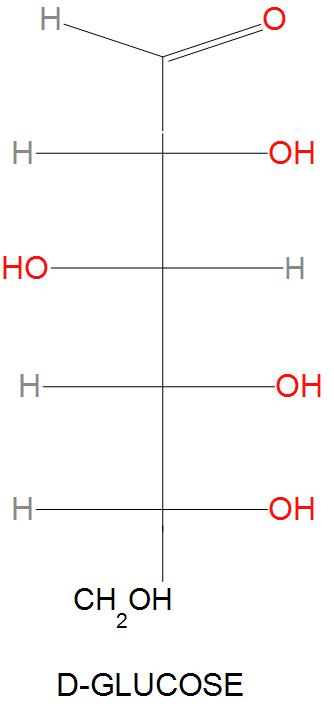
- D-Glucose rearranges to D-fructose. It is found that D-glucose (an aldose ) on further reaction with $O{{H}^{-}}$, Enol changes into keto form. The initial deprotonation takes place at a carbon atom which is a stereocenter. Hence, this was called D-fructose that has a keto group. D-fructose will have following structure:
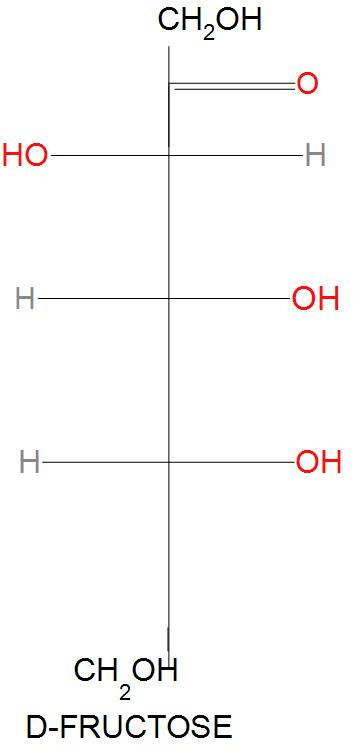
- This on further reaction with $O{{H}^{-}}$ will change the keto group into enol group. And D-mannose will be formed. Or we can say that in the given chemical reaction the enol is protonated from two faces, which results in the back formation of the epimer D-mannose. D-mannose is actually an epimer of D-glucose, this because these two sugars differ only in the configuration at C-2 position.
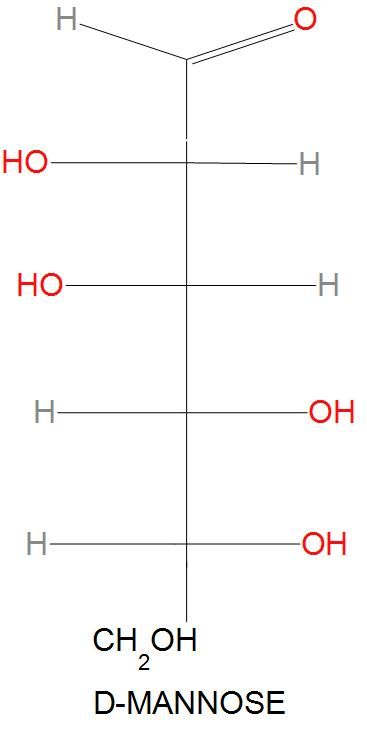
- Hence, we can conclude that the correct option is (B), that is A and B are D-mannose and D-Fructose.
So, the correct answer is “Option B”.
Note: - This reaction was relevant for the industrial production of ketone.
- It is found that Fructose is found to be more damaging to health than that of glucose.
- It is found that having too much glucose in the blood for a long time can cause serious health problems if it is treated. Hyperglycaemia is found to damage the vessels that can supply the blood to various organs, and this can increase the risk of kidney disease, heart disease and nerve problems.
Complete step by step answer:
- The reaction given here is called the Lobry de Bruyn-van Ekenstein reaction. We will write the reaction as: $D-glu\cos e\xrightarrow{O{{H}^{-}}}D-mannose+D-Fructose$
- Structurally, we can represent it as:

- Firstly, we are being given with D-Glucose. The structure of D-Glucose is:

- D-Glucose rearranges to D-fructose. It is found that D-glucose (an aldose ) on further reaction with $O{{H}^{-}}$, Enol changes into keto form. The initial deprotonation takes place at a carbon atom which is a stereocenter. Hence, this was called D-fructose that has a keto group. D-fructose will have following structure:

- This on further reaction with $O{{H}^{-}}$ will change the keto group into enol group. And D-mannose will be formed. Or we can say that in the given chemical reaction the enol is protonated from two faces, which results in the back formation of the epimer D-mannose. D-mannose is actually an epimer of D-glucose, this because these two sugars differ only in the configuration at C-2 position.

- Hence, we can conclude that the correct option is (B), that is A and B are D-mannose and D-Fructose.
So, the correct answer is “Option B”.
Note: - This reaction was relevant for the industrial production of ketone.
- It is found that Fructose is found to be more damaging to health than that of glucose.
- It is found that having too much glucose in the blood for a long time can cause serious health problems if it is treated. Hyperglycaemia is found to damage the vessels that can supply the blood to various organs, and this can increase the risk of kidney disease, heart disease and nerve problems.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




