
D-glucose and L-glucose differ in:
(A) Configuration at the highest number chiral carbon.
(B) Configuration at first chiral carbon
(C) Configuration at each chiral carbon
(D) Configuration at the second chiral carbon.
Answer
598.5k+ views
Hint: Try to recall that D and L are not related with the optical rotation but they indicate their configuration and are used to differentiate between two different shapes of glucose molecules. Now, by using it you can easily find the correct option from the given ones.
Complete step by step solution:
* It is known to you that “D” and “L” specifications in the name of D-glucose and L-glucose are used to differentiate between two shapes of glucose molecules.
* D-glucose and L-glucose are enantiomers meaning that their molecular structures are mirror images of each other.
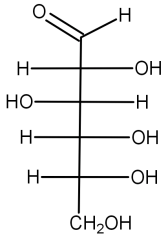
* The structure of D-glucose is given below:
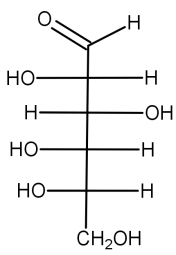
* The structure of L-glucose is shown below:
* D-glucose: In the glucose molecule, an oxygen and hydrogen atom is bonded to carbon atom. On the other end of the glucose molecule, there is a double-bonded oxygen atom. Looking at the Fischer projection of D-glucose with the double-bonded oxygen atom pointed down, the oxygen and hydrogen group at the top of the atom points to the right.
* L-glucose: D-glucose and L-glucose are made up of the same atoms. The only difference between the two structures is displayed through fischer projection. Unlike D-glucose, the oxygen and hydrogen group of atoms in L-glucose points to the left in fischer projection. If these two molecules faced each other, they would look like a reflection of one another.
Therefore, from above we can conclude that option C is the correct option to the given question.
Note:
* It should be remembered that D-glucose is naturally occurring while L-glucose does not occur naturally but can be synthesised in the laboratory.
* Also, you should remember that L-glucose cannot be used by living organisms because it cannot be phosphorylated by hexokinase.
Complete step by step solution:
* It is known to you that “D” and “L” specifications in the name of D-glucose and L-glucose are used to differentiate between two shapes of glucose molecules.
* D-glucose and L-glucose are enantiomers meaning that their molecular structures are mirror images of each other.
* The structure of D-glucose is given below:

* The structure of L-glucose is shown below:

* D-glucose: In the glucose molecule, an oxygen and hydrogen atom is bonded to carbon atom. On the other end of the glucose molecule, there is a double-bonded oxygen atom. Looking at the Fischer projection of D-glucose with the double-bonded oxygen atom pointed down, the oxygen and hydrogen group at the top of the atom points to the right.
* L-glucose: D-glucose and L-glucose are made up of the same atoms. The only difference between the two structures is displayed through fischer projection. Unlike D-glucose, the oxygen and hydrogen group of atoms in L-glucose points to the left in fischer projection. If these two molecules faced each other, they would look like a reflection of one another.
Therefore, from above we can conclude that option C is the correct option to the given question.
Note:
* It should be remembered that D-glucose is naturally occurring while L-glucose does not occur naturally but can be synthesised in the laboratory.
* Also, you should remember that L-glucose cannot be used by living organisms because it cannot be phosphorylated by hexokinase.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




