
What determines an ${{S}_{N}}1$ reaction rate?
Answer
530.7k+ views
Hint: In a substitution reaction, from a compound, one substituent will leave the compound and another substituent will get attached at that place. So, the nature of the bond of the reactant and incoming nucleophile affects the substitution reaction.
Complete answer:
${{S}_{N}}1$ reaction means Unimolecular Nucleophilic substitution reaction and it occurs in two steps. As we know that in a substitution reaction, from a compound one substituent will leave the compound and another substituent will get attached at that place. So, the incoming substituent that will attack the reactant will be nucleophile.
So, the nature of the bond of the reactant and incoming nucleophile affects the substitution reaction. since, the ${{S}_{N}}1$ reaction takes place in two steps, i.e, in the first step the substituent will leave the reactant molecule forming a carbocation then in the second step the nucleophile will attack the carbocation and will form the product.
Therefore, in ${{S}_{N}}1$ reaction the factor that determines the rate of reaction will be the nature of the bond between the leaving nucleophile and carbon atom of the reactant. If the bond is weak then it will break easily and the first reaction will take place fast and then the second step will take place easily.
Since the first step contains only one reactant, then the rate of the reaction will depend only on one reactant molecule. The general reaction is given below:
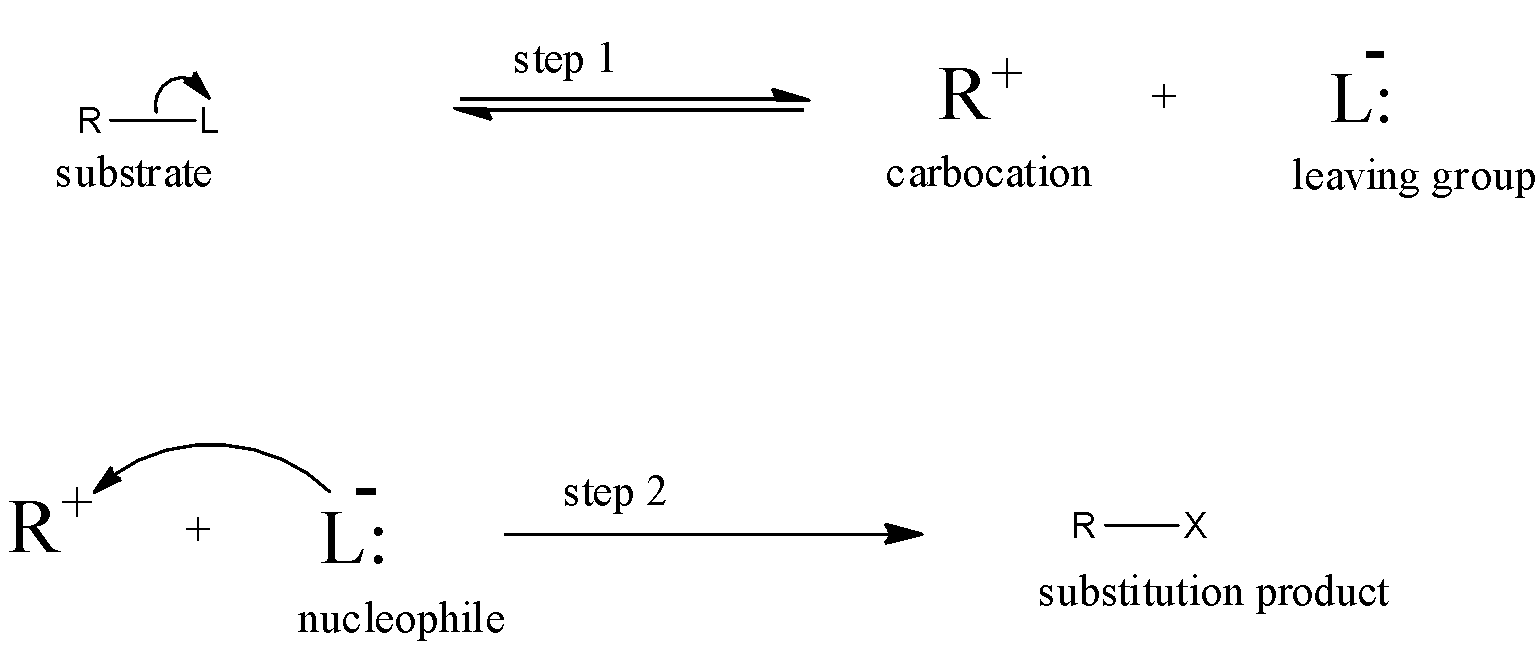
Note:
In the ${{S}_{N}}2$ reaction, the nature of the incoming nucleophile will also determine the rate of the reaction because in the first step only the leaving group will leave the reactant and the incoming nucleophile will attack the carbocation.
Complete answer:
${{S}_{N}}1$ reaction means Unimolecular Nucleophilic substitution reaction and it occurs in two steps. As we know that in a substitution reaction, from a compound one substituent will leave the compound and another substituent will get attached at that place. So, the incoming substituent that will attack the reactant will be nucleophile.
So, the nature of the bond of the reactant and incoming nucleophile affects the substitution reaction. since, the ${{S}_{N}}1$ reaction takes place in two steps, i.e, in the first step the substituent will leave the reactant molecule forming a carbocation then in the second step the nucleophile will attack the carbocation and will form the product.
Therefore, in ${{S}_{N}}1$ reaction the factor that determines the rate of reaction will be the nature of the bond between the leaving nucleophile and carbon atom of the reactant. If the bond is weak then it will break easily and the first reaction will take place fast and then the second step will take place easily.
Since the first step contains only one reactant, then the rate of the reaction will depend only on one reactant molecule. The general reaction is given below:

Note:
In the ${{S}_{N}}2$ reaction, the nature of the incoming nucleophile will also determine the rate of the reaction because in the first step only the leaving group will leave the reactant and the incoming nucleophile will attack the carbocation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




