
How do I determine the molecular shape of a molecule?
Answer
574.2k+ views
Hint: The answer is based on the various theories that are proposed to determine the structure of a molecule and among those theories one theory predicts the shape of the molecule based on the valence shell and the required answer lies in this fact.
Complete answer:
In the classes of chemistry, we are familiar with the theories that are proposed for the prediction of geometry of the molecule and also about the number of bonds that are present between the atoms of a molecule.
Now let us see which theory among these predicts the shape of the molecule.
- Usually in the molecule, the shape of the molecule can be determined by finding the hybridisation of the molecule and also the presence of a lone pair of electrons on the atom.
- The theory which predicts the shape based on this data is the valence shell electron pair repulsion theory which is abbreviated as VSEPR theory.
- This theory predicts the shape mainly based on the number of electron pairs surrounding their central atoms.
Let us take an example of a water molecule. It has the hybridisation of $s{{p}^{3}}$ and the oxygen has two lone pairs of electrons.
Water has two hydrogen atoms bonded to central oxygen atom and the shape of the molecule according to the VSEPR theory is as shown below,
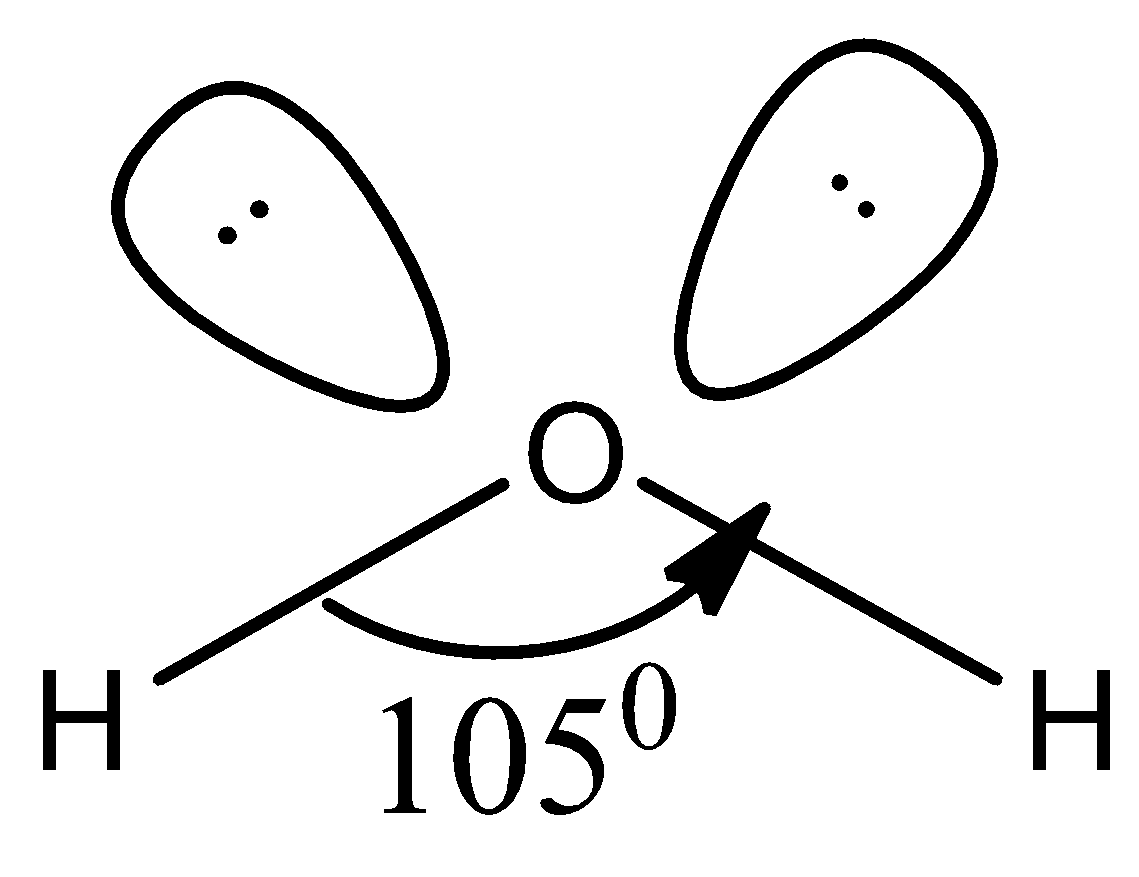
Accordingly, because of the lone pair of electrons present, the bond angle slightly changes from usual ${{109.5}^{0}}$ to ${{105}^{0}}$ and therefore, the shape of the molecule is bent structure.
In this way, we can find the shape of the molecule using VSEPR theory.
Note:
Note that the shape of the molecule is dependent on the repulsion between the lone pair and the bond pair and also with each other which follows the trend of decreasing repulsion as lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.
Complete answer:
In the classes of chemistry, we are familiar with the theories that are proposed for the prediction of geometry of the molecule and also about the number of bonds that are present between the atoms of a molecule.
Now let us see which theory among these predicts the shape of the molecule.
- Usually in the molecule, the shape of the molecule can be determined by finding the hybridisation of the molecule and also the presence of a lone pair of electrons on the atom.
- The theory which predicts the shape based on this data is the valence shell electron pair repulsion theory which is abbreviated as VSEPR theory.
- This theory predicts the shape mainly based on the number of electron pairs surrounding their central atoms.
Let us take an example of a water molecule. It has the hybridisation of $s{{p}^{3}}$ and the oxygen has two lone pairs of electrons.
Water has two hydrogen atoms bonded to central oxygen atom and the shape of the molecule according to the VSEPR theory is as shown below,

Accordingly, because of the lone pair of electrons present, the bond angle slightly changes from usual ${{109.5}^{0}}$ to ${{105}^{0}}$ and therefore, the shape of the molecule is bent structure.
In this way, we can find the shape of the molecule using VSEPR theory.
Note:
Note that the shape of the molecule is dependent on the repulsion between the lone pair and the bond pair and also with each other which follows the trend of decreasing repulsion as lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




