
Determine the incorrect statements using the given phase diagram of water.
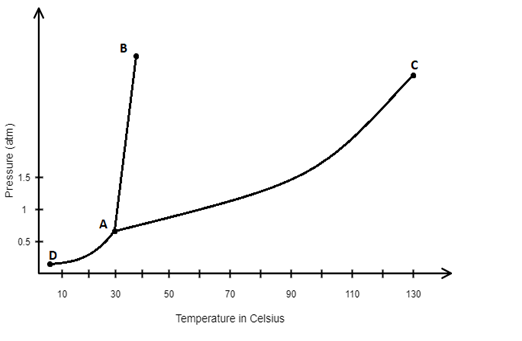
A. The triple point is point A. This is the point at which three phases are in equilibrium with one another.
B. The line AB is the line representing the solid – liquid equilibrium line. Anywhere along this line the substance could melt or freeze.
C. The slope of line AB is negative. This slope indicates that the solid is much denser than the liquid.
D. Line AD represents the phase changes of sublimation and deposition
E. Line AC represents where the substance would condense and vaporize.

Answer
566.7k+ views
Hint: Before talking about the answer, you should know what a phase diagram is. It is defined as a chart where different phases of compound co-exist at equilibrium. You should also have an idea about triple points. It is defined as a point where solid, liquid and gases co-exist at equilibrium.
Complete step by step answer:
A phase diagram is defined as a kind of a chart where the different phases of the compound co-exist at equilibrium under certain conditions like temperature, pressure, volume.
In this question, here it is given the phase diagram of water in which it consists of three phases, that is, solid, liquid and gas.
One of the special properties is that the water in solid form (ice) is less dense than water in liquid form just above the freezing point. Here, the triple point is point A. Triple point is defined as the point where all three phases stay in equilibrium with each other. In this question solid, liquid and gases co exist at point A. Therefore option A is correct.
The line AB is the line that represents the solid – liquid equilibrium. The substance can freeze or melt anywhere along this line. at line AD, the mass of water and ice remain the same which indicates solid liquid equilibrium because the rate at which the molecules transfer from solid to liquid are equal. Therefore option B is correct.
Here, the line AD represents the phase changes of deposition and sublimation. Sublimation is a process where a molecule changes from gaseous phase to solid phase and settlement process of gaseous phase into solid phase is known as deposition. Therefore, option D is correct.
The condensation and vaporization of the substance represents at line AC. Condensation is a process that converts gaseous into liquid and vapourisation is a process that converts liquid phase to vapour. Hence option E is correct.
Therefore, the option (C) is the incorrect statement using the given phase diagram of water. Because in AD the pressure is increasing whereas the melting temperature is decreasing. Hence the ice is less dense than water.
Note: While writing the coefficients of a balanced chemical equation, we never write the coefficient one.
It is necessary to write the coefficients of the chemical equation as lowest possible ratio as it will provide a more clear detail, as to how many whole atoms are involved in the reaction.
Complete step by step answer:
A phase diagram is defined as a kind of a chart where the different phases of the compound co-exist at equilibrium under certain conditions like temperature, pressure, volume.
In this question, here it is given the phase diagram of water in which it consists of three phases, that is, solid, liquid and gas.
One of the special properties is that the water in solid form (ice) is less dense than water in liquid form just above the freezing point. Here, the triple point is point A. Triple point is defined as the point where all three phases stay in equilibrium with each other. In this question solid, liquid and gases co exist at point A. Therefore option A is correct.
The line AB is the line that represents the solid – liquid equilibrium. The substance can freeze or melt anywhere along this line. at line AD, the mass of water and ice remain the same which indicates solid liquid equilibrium because the rate at which the molecules transfer from solid to liquid are equal. Therefore option B is correct.
Here, the line AD represents the phase changes of deposition and sublimation. Sublimation is a process where a molecule changes from gaseous phase to solid phase and settlement process of gaseous phase into solid phase is known as deposition. Therefore, option D is correct.
The condensation and vaporization of the substance represents at line AC. Condensation is a process that converts gaseous into liquid and vapourisation is a process that converts liquid phase to vapour. Hence option E is correct.
Therefore, the option (C) is the incorrect statement using the given phase diagram of water. Because in AD the pressure is increasing whereas the melting temperature is decreasing. Hence the ice is less dense than water.
Note: While writing the coefficients of a balanced chemical equation, we never write the coefficient one.
It is necessary to write the coefficients of the chemical equation as lowest possible ratio as it will provide a more clear detail, as to how many whole atoms are involved in the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




