
How would you determine the formula and molar mass Aluminium sulfide?
Answer
558.6k+ views
Hint Molar mass is the sum of the individual atoms present in a compound. The Aluminium is a trivalent ion and the Sulphide has a valency of -2.
Complete step by step solution:
So in the question there are two parts: first one is asked how we will determine molecular formulae and the second part is molar mass.
So now let’s first solve the part one i.e. to write the chemical formulae of the given compound. From the lower classes we are studying about the periodic table and we know that we represent each element with each notation with respect to their names.
So here we have to write the formulae of Aluminium sulfide, we should know how the ions in them are represented.
In aluminum sulphide, there is one anion and a cation. The cation is the trivalent aluminium ion, we know that aluminium is represented as Al and since it produces a trivalent ion, the aluminium ion has a formula of $A{{l}^{3+}}$.
Now we have to know about the anions, here sulphide is the anion and it has a charge of-2, sulphide is represented as S. Therefore the sulphide ion is represented as ${{S}^{2-}}$.
Now we have to write the equation. For that we will write the respective ions and we know that a compound will be neutral. So we will neutralize the charge of the compound by multiplying the appropriate numerical digit with the ions in such a fashion that the charge gets neutralized and the multiplied number is written as the subscript.
So let's see how the charge is neutralized,
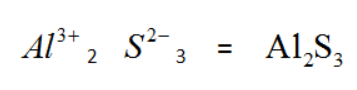
Here we have multiplied with $A{{l}^{3+}}$ and 3 with ${{S}^{2-}}$, which neutralizes the charge of the compound and the final formulae for the compound is obtained.
So the molecular formulae of aluminium sulphide is $A{{l}_{2}}{{S}_{3}}$
Now let’s move to the second part of the question which is to find the molar mass of the compound. Molar mass is the total sum of the atomic masses of the individual atoms present in the compound.
We know that the Aluminium has a atomic mass of $\text{26}\text{.98g/mol}$ and Sulphide has a atomic mass number $\text{32}\text{.06g/mol}$
Now let’s calculate the molar mass for $A{{l}_{2}}{{S}_{3}}$, since there are 2 Al atoms and 3 S atoms in the compound the atomic mass of Al should be added twice and the atomic mass of S should be added thrice.
$\text{Molar}\,\text{mass}\,\text{of}\,\text{A}{{\text{l}}_{\text{2}}}{{\text{S}}_{\text{3}}}\text{=2 }\!\!\times\!\!\text{ }\left( \text{26}\text{.98} \right)\text{+3 }\!\!\times\!\!\text{ }\left( \text{32}\text{.06} \right)$
$\text{Molar}\,\text{mass}\,\text{of}\,\text{A}{{\text{l}}_{\text{2}}}{{\text{S}}_{\text{3}}}\text{=53}\text{.96+96}\text{.18=150}\text{.14g/mol}$
Note: We could find the molecular formula of the compound by simply crisscrossing the charge of the ions present, but the ultimate concept of doing so is to neutralize the ionic charge and to get a neutral compound.
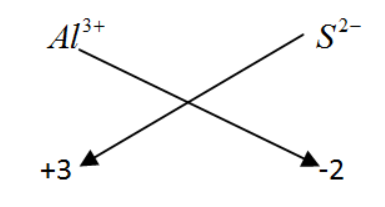
While writing the molecular formulae we will only consider the chare and not the sign associated with the ions.
Complete step by step solution:
So in the question there are two parts: first one is asked how we will determine molecular formulae and the second part is molar mass.
So now let’s first solve the part one i.e. to write the chemical formulae of the given compound. From the lower classes we are studying about the periodic table and we know that we represent each element with each notation with respect to their names.
So here we have to write the formulae of Aluminium sulfide, we should know how the ions in them are represented.
In aluminum sulphide, there is one anion and a cation. The cation is the trivalent aluminium ion, we know that aluminium is represented as Al and since it produces a trivalent ion, the aluminium ion has a formula of $A{{l}^{3+}}$.
Now we have to know about the anions, here sulphide is the anion and it has a charge of-2, sulphide is represented as S. Therefore the sulphide ion is represented as ${{S}^{2-}}$.
Now we have to write the equation. For that we will write the respective ions and we know that a compound will be neutral. So we will neutralize the charge of the compound by multiplying the appropriate numerical digit with the ions in such a fashion that the charge gets neutralized and the multiplied number is written as the subscript.
So let's see how the charge is neutralized,
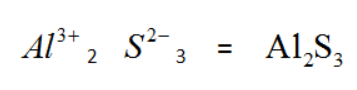
Here we have multiplied with $A{{l}^{3+}}$ and 3 with ${{S}^{2-}}$, which neutralizes the charge of the compound and the final formulae for the compound is obtained.
So the molecular formulae of aluminium sulphide is $A{{l}_{2}}{{S}_{3}}$
Now let’s move to the second part of the question which is to find the molar mass of the compound. Molar mass is the total sum of the atomic masses of the individual atoms present in the compound.
We know that the Aluminium has a atomic mass of $\text{26}\text{.98g/mol}$ and Sulphide has a atomic mass number $\text{32}\text{.06g/mol}$
Now let’s calculate the molar mass for $A{{l}_{2}}{{S}_{3}}$, since there are 2 Al atoms and 3 S atoms in the compound the atomic mass of Al should be added twice and the atomic mass of S should be added thrice.
$\text{Molar}\,\text{mass}\,\text{of}\,\text{A}{{\text{l}}_{\text{2}}}{{\text{S}}_{\text{3}}}\text{=2 }\!\!\times\!\!\text{ }\left( \text{26}\text{.98} \right)\text{+3 }\!\!\times\!\!\text{ }\left( \text{32}\text{.06} \right)$
$\text{Molar}\,\text{mass}\,\text{of}\,\text{A}{{\text{l}}_{\text{2}}}{{\text{S}}_{\text{3}}}\text{=53}\text{.96+96}\text{.18=150}\text{.14g/mol}$
Note: We could find the molecular formula of the compound by simply crisscrossing the charge of the ions present, but the ultimate concept of doing so is to neutralize the ionic charge and to get a neutral compound.

While writing the molecular formulae we will only consider the chare and not the sign associated with the ions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




