
How can I determine NMR splitting pattern?
Answer
565.2k+ views
Hint As we know that NMR gives us the information about how many how much neighbouring hydrogens exit for an individual or for a group of hydrogen atoms. It is basically found that if there is one hydrogen present on the adjacent atoms, then resonance will split into two peaks having equal size.
Complete answer:
- In order to find the NMR splitting pattern in hydrogen atoms, we will first count the adjacent hydrogen atoms present, and then will add one to that number. Let us consider an example of $C{{H}_{2}}ClC{{H}_{3}}$, we will first draw its structure as:
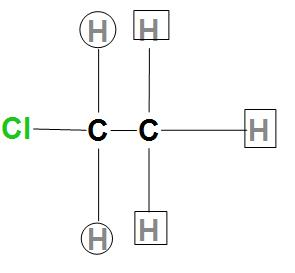
- Here, we can see that there are three identical hydrogen atoms that are marked as square, and two hydrogen atoms that are circled are adjacent to the square hydrogens. So, they are found to exhibit a quartet that is (4 peak, 3+1) splitting pattern.
- For the square hydrogen, we can see they are adjacent to the two same hydrogens that are circled, so it is found that their splitting pattern is triplet.
Note:
- As we know that if there were no hydrogens present on the adjacent hydrogen atoms, then the resonance will remain a single peak.
- And if there were two hydrogens present on the adjacent hydrogen atoms then the resonance will split into two peaks having equal size, called doublet.
- Whereas, if there were three hydrogens present on the adjacent hydrogen atoms then the resonance will split into three peaks having equal size, called a triplet.
Complete answer:
- In order to find the NMR splitting pattern in hydrogen atoms, we will first count the adjacent hydrogen atoms present, and then will add one to that number. Let us consider an example of $C{{H}_{2}}ClC{{H}_{3}}$, we will first draw its structure as:

- Here, we can see that there are three identical hydrogen atoms that are marked as square, and two hydrogen atoms that are circled are adjacent to the square hydrogens. So, they are found to exhibit a quartet that is (4 peak, 3+1) splitting pattern.
- For the square hydrogen, we can see they are adjacent to the two same hydrogens that are circled, so it is found that their splitting pattern is triplet.
Note:
- As we know that if there were no hydrogens present on the adjacent hydrogen atoms, then the resonance will remain a single peak.
- And if there were two hydrogens present on the adjacent hydrogen atoms then the resonance will split into two peaks having equal size, called doublet.
- Whereas, if there were three hydrogens present on the adjacent hydrogen atoms then the resonance will split into three peaks having equal size, called a triplet.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




