
Designate the double bonds as E, Z, or none (N) configuration in the boxes provided below.
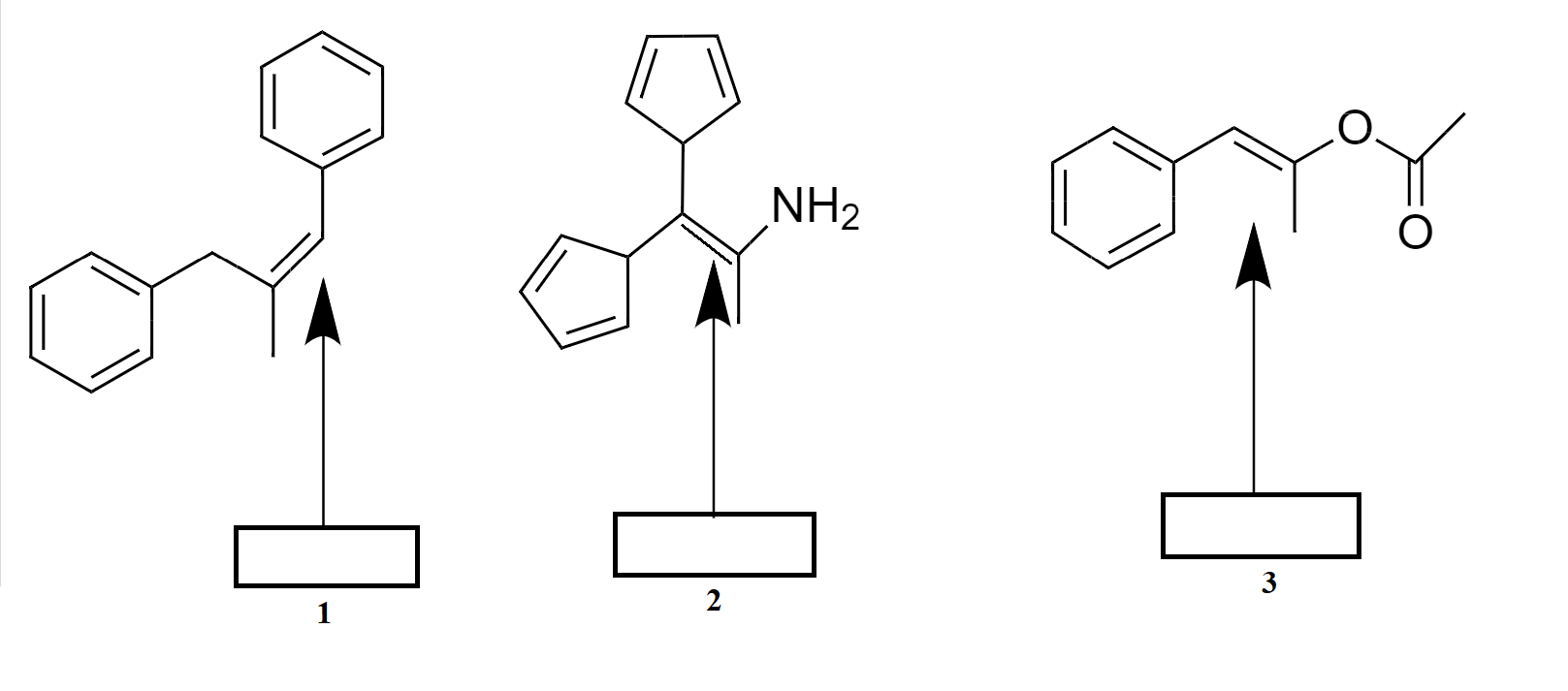
A.1-Z, 2-N, 3-Z
B.1-E, 2-N, 3-E
C.1-Z, 2-N, 3-E
D.None of these

Answer
531.6k+ views
Hint: Before solving this question, we should first know the E, Z, and N configuration. After understanding each configuration we will be able to answer this question.
Complete answer:
Cis and trans stereoisomerism can be distinguished with the condition that if the two same alkyl groups $C=C$ are on the same side of the opposite side of the double bond.
This theory only works if there is a double bond between them. This is the limitation of cis and trans theory so for a molecule that has four different groups, we cannot use this theory as they are not identical. For this, We use the E and Z configuration.
For this, we need to look at each group separately and the motive is to sort which two groups on each carbon hold the priority. Then see Does the higher priority group on each carbon is on the same side or the opposite of the double bond. If the alkyl groups are pointing up and down, they are on opposite sides and the configuration would be E whereas if the groups are on the same side then the configuration would be Z.
E and Z are stereoisomers just like cis and trans as they do not mirror images of each other.
When the two identical groups are there on a single carbon of the double bond. That is N configuration.
So, Option (C) 1-Z, 2-N, 3-E is correct.
Note:
E configuration – Priority Groups are present on the opposite side of the double bond.
Z configuration- Priority Groups are present on the same side of the double bond.
N configuration – Two identical groups are present on the single carbon of the double bond.
Complete answer:
Cis and trans stereoisomerism can be distinguished with the condition that if the two same alkyl groups $C=C$ are on the same side of the opposite side of the double bond.
This theory only works if there is a double bond between them. This is the limitation of cis and trans theory so for a molecule that has four different groups, we cannot use this theory as they are not identical. For this, We use the E and Z configuration.
For this, we need to look at each group separately and the motive is to sort which two groups on each carbon hold the priority. Then see Does the higher priority group on each carbon is on the same side or the opposite of the double bond. If the alkyl groups are pointing up and down, they are on opposite sides and the configuration would be E whereas if the groups are on the same side then the configuration would be Z.
E and Z are stereoisomers just like cis and trans as they do not mirror images of each other.
When the two identical groups are there on a single carbon of the double bond. That is N configuration.
So, Option (C) 1-Z, 2-N, 3-E is correct.
Note:
E configuration – Priority Groups are present on the opposite side of the double bond.
Z configuration- Priority Groups are present on the same side of the double bond.
N configuration – Two identical groups are present on the single carbon of the double bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




