
Describe two chemical tests that can be used to distinguish between the following pair of compounds? PLEASE, WRITE OUT THE EQUATIONS, INCLUDING THE REAGENTS AND THE PRODUCTS. i) Butane and 2-butene.
Answer
491.1k+ views
Hint: To solve this question we will use two tests by which we can differentiate both butane and 2-butene. The two tests are :
-Bromine decolourization test
-Baeyer’s unsaturation test
Complete answer:
To solve this question, we will discuss both tests one by one which are the bromine decolourization test and Baeyer unsaturation tests.
Test-1: Bromine decolourization test:
The bromine test is a qualitative test used for the presence of unsaturated carbon, phenols and anilines. A sample is treated with a small amount of bromine in an organic solvent, being as dichloromethane or carbon tetrachloride. When unsaturated carbon or phenol or aniline in the sample reacts with bromine the deep brown colour of bromine disappears. The more unsaturated carbon in the sample is the more bromine it reacts with and hence lesser coloured the solution will appear.
By adding a few drops of $ B{r_2} $ solution in $ CC{l_4} $ to a solution of each hydrocarbon in $ CC{l_4} $ . The butane will give no reaction whereas 2-butene will decolourize the bromine solution.
When butane is react with Bromine:
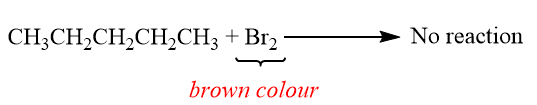
When 2-butene is react with Bromine:
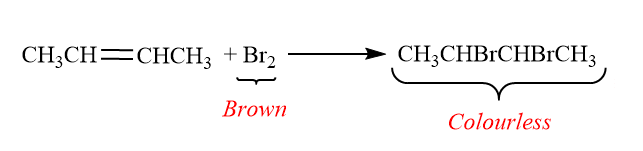
Test-2: Baeyer unsaturation test:
The Baeyer unsaturation test is used for determination of the presence of carbon-carbon double bonded compounds which are known as alkenes or carbon-carbon triple bonded compounds known as alkynes. It is the test for unsaturated compounds in which potassium permanganate is used.
In this test we use dilute Potassium Permanganate to oxidize the carbon-carbon double bond or triple bond. It is known as oxidation because the double bond is replaced by a hydroxyl group, i.e. an $ OH $ group. The carbon’s oxidation number goes from $ + 1 $ to $ + 2 $ , thus it loses an electron. The butane will give no colour change whereas 2-butene will give dark brown precipitate.
The reaction has shown below:
When butane is react with potassium permanganate:
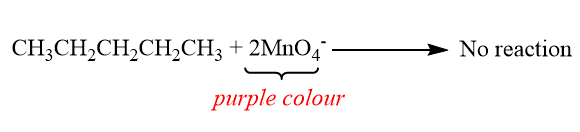
When 2-butene is react with potassium permanganate:
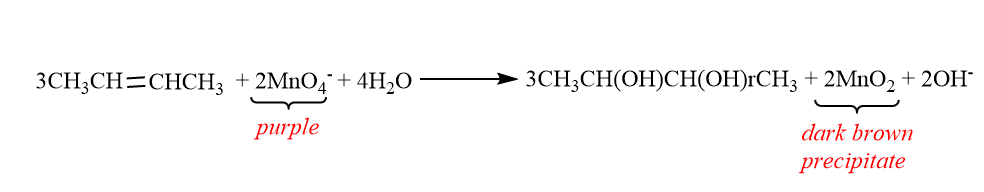
Note:
Bromine decolourization is used as a simple qualitative test for unsaturated carbons. Bromine is a dark red-brown liquid, but alkenes and dibromo alkanes are colourless. So, dilute solution of bromine in inert colourless solvent like dichloromethane is rapidly decolourized when it is added to an alkene.
-Bromine decolourization test
-Baeyer’s unsaturation test
Complete answer:
To solve this question, we will discuss both tests one by one which are the bromine decolourization test and Baeyer unsaturation tests.
Test-1: Bromine decolourization test:
The bromine test is a qualitative test used for the presence of unsaturated carbon, phenols and anilines. A sample is treated with a small amount of bromine in an organic solvent, being as dichloromethane or carbon tetrachloride. When unsaturated carbon or phenol or aniline in the sample reacts with bromine the deep brown colour of bromine disappears. The more unsaturated carbon in the sample is the more bromine it reacts with and hence lesser coloured the solution will appear.
By adding a few drops of $ B{r_2} $ solution in $ CC{l_4} $ to a solution of each hydrocarbon in $ CC{l_4} $ . The butane will give no reaction whereas 2-butene will decolourize the bromine solution.
When butane is react with Bromine:
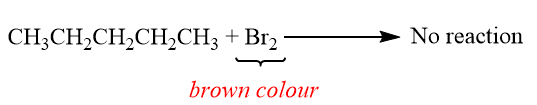
When 2-butene is react with Bromine:

Test-2: Baeyer unsaturation test:
The Baeyer unsaturation test is used for determination of the presence of carbon-carbon double bonded compounds which are known as alkenes or carbon-carbon triple bonded compounds known as alkynes. It is the test for unsaturated compounds in which potassium permanganate is used.
In this test we use dilute Potassium Permanganate to oxidize the carbon-carbon double bond or triple bond. It is known as oxidation because the double bond is replaced by a hydroxyl group, i.e. an $ OH $ group. The carbon’s oxidation number goes from $ + 1 $ to $ + 2 $ , thus it loses an electron. The butane will give no colour change whereas 2-butene will give dark brown precipitate.
The reaction has shown below:
When butane is react with potassium permanganate:
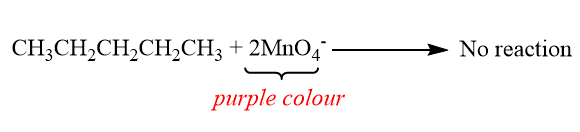
When 2-butene is react with potassium permanganate:

Note:
Bromine decolourization is used as a simple qualitative test for unsaturated carbons. Bromine is a dark red-brown liquid, but alkenes and dibromo alkanes are colourless. So, dilute solution of bromine in inert colourless solvent like dichloromethane is rapidly decolourized when it is added to an alkene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




