
Derive mathematical expression for first law of thermodynamics?
Answer
586.5k+ views
Hint:From thermodynamics first law, the total energy associated with a system is constant, but its form can be changed. In other words, we can say that the work done by a system in a cyclic process is equal to the heat supplied to it.
Complete step by step answer:
Let us consider a system undergoing a process from state first to state second by following the path ‘a’ and returns from state second to state first by following path ‘b’ and path ‘c’ as shown below.
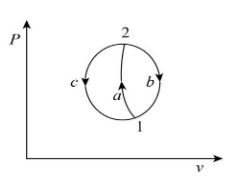
From the concept of the first law of thermodynamics, for the cyclic process 1-a-2-b-1 we can write:
\[\oint_{1 - a - 2 - b - 1} {\left( {Q - W} \right)} = 0\]
We can break the above cyclic process into two processes 1-a-2 and 2-b-1, that is,
\[\int_{1 - a - 2} {\left( {Q - W} \right)} + \int_{2 - b - 1} {\left( {Q - W} \right)} = 0\]……(1)
Process 1-a-2-c-1 is also a cyclic process so that we can write:
\[\oint_{1 - a - 2 - c - 1} {\left( {Q - W} \right)} = 0\]
We can break the above cyclic process into process 1-a-2 and process 2-c-1.
\[\int_{1 - a - 2} {\left( {Q - W} \right)} + \int_{2 - c - 1} {\left( {Q - W} \right)} = 0\]……(2)
On comparing the equation (1) and equation (2), we can write:
\[\int_{2 - b - 1} {\left( {Q - W} \right)} = \int_{2 - c - 1} {\left( {Q - W} \right)} \]
By closely observing equation (1) and equation (2) we can conclude that the value of the above expression is non-zero.
\[\begin{array}{l}
\int_{2 - b - 1} {\left( {Q - W} \right)} = \int_{2 - c - 1} {\left( {Q - W} \right)} \\
\ne 0
\end{array}\]
Based on the above expression, we can say that the term \[\int_{2 - b - 1} {\left( {Q - W} \right)} \] and term \[\int_{2 - c - 1} {\left( {Q - W} \right)} \] have some value which is independent of path. Hence, it is a point function.
We know that point functions are properties of a thermodynamic system which is an exact differential, and this non-zero term is defined as the energy of the system.
\[\begin{array}{l}
\int_{2 - b - 1} {\left( {Q - W} \right)} = \int_{2 - c - 1} {\left( {Q - W} \right)} \\= dE
\end{array}\]
By generalising the above expression for a non-cyclic process, we can write:
\[\begin{array}{l}
Q - W = dE\\
Q = W + dE
\end{array}\]
Therefore, the mathematical relationship of the first law of thermodynamics for a non-cyclic process is \[Q = W + dE\].
Note: In this problem, we can note that the state of a system is a point function, hence exact differential. We know that exact differential are properties of the system that’s why we defined a point function as the property of the system.
Complete step by step answer:
Let us consider a system undergoing a process from state first to state second by following the path ‘a’ and returns from state second to state first by following path ‘b’ and path ‘c’ as shown below.

From the concept of the first law of thermodynamics, for the cyclic process 1-a-2-b-1 we can write:
\[\oint_{1 - a - 2 - b - 1} {\left( {Q - W} \right)} = 0\]
We can break the above cyclic process into two processes 1-a-2 and 2-b-1, that is,
\[\int_{1 - a - 2} {\left( {Q - W} \right)} + \int_{2 - b - 1} {\left( {Q - W} \right)} = 0\]……(1)
Process 1-a-2-c-1 is also a cyclic process so that we can write:
\[\oint_{1 - a - 2 - c - 1} {\left( {Q - W} \right)} = 0\]
We can break the above cyclic process into process 1-a-2 and process 2-c-1.
\[\int_{1 - a - 2} {\left( {Q - W} \right)} + \int_{2 - c - 1} {\left( {Q - W} \right)} = 0\]……(2)
On comparing the equation (1) and equation (2), we can write:
\[\int_{2 - b - 1} {\left( {Q - W} \right)} = \int_{2 - c - 1} {\left( {Q - W} \right)} \]
By closely observing equation (1) and equation (2) we can conclude that the value of the above expression is non-zero.
\[\begin{array}{l}
\int_{2 - b - 1} {\left( {Q - W} \right)} = \int_{2 - c - 1} {\left( {Q - W} \right)} \\
\ne 0
\end{array}\]
Based on the above expression, we can say that the term \[\int_{2 - b - 1} {\left( {Q - W} \right)} \] and term \[\int_{2 - c - 1} {\left( {Q - W} \right)} \] have some value which is independent of path. Hence, it is a point function.
We know that point functions are properties of a thermodynamic system which is an exact differential, and this non-zero term is defined as the energy of the system.
\[\begin{array}{l}
\int_{2 - b - 1} {\left( {Q - W} \right)} = \int_{2 - c - 1} {\left( {Q - W} \right)} \\= dE
\end{array}\]
By generalising the above expression for a non-cyclic process, we can write:
\[\begin{array}{l}
Q - W = dE\\
Q = W + dE
\end{array}\]
Therefore, the mathematical relationship of the first law of thermodynamics for a non-cyclic process is \[Q = W + dE\].
Note: In this problem, we can note that the state of a system is a point function, hence exact differential. We know that exact differential are properties of the system that’s why we defined a point function as the property of the system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




