
Define the reference electrode.
Answer
593.7k+ views
Hint: Standard or normal hydrogen electrode is used as a reference electrode. It is used to calculate the absolute value of the electrode potential of a single electrode.
Complete answer:
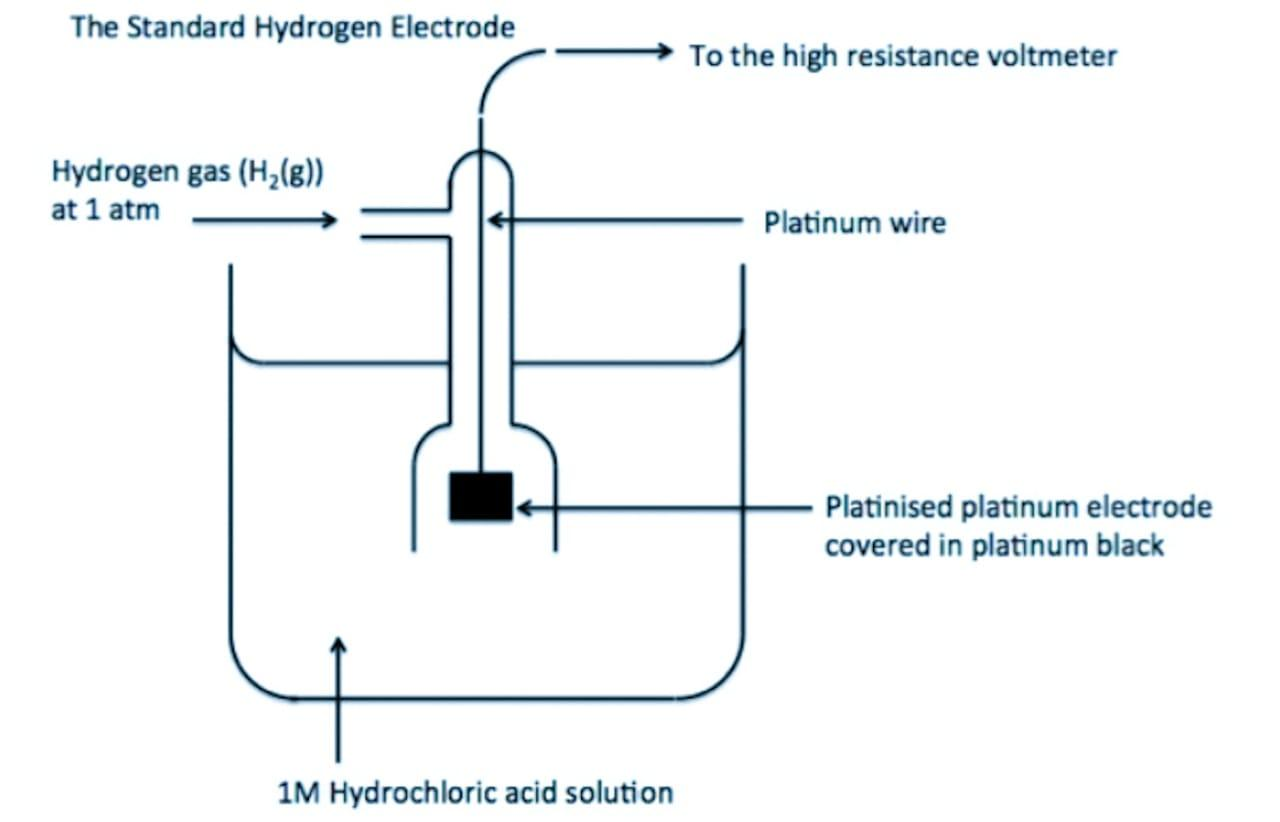
The absolute value of the electrode potential of a single electrode called a single electrode potential cannot be determined because the oxidation half-reaction or reduction half-reaction cannot take place alone. It can only be measured by using some electrode as a reference electrode. The reference electrode used is the standard or normal hydrogen electrode (S.H.E or N.H.E). In the standard hydrogen gas electrode, hydrogen gas at 1 bar pressure is passed into 1 M HCl at 298 K in which a foil of platinum-coated with platinum black (finely divided platinum) remains immersed. It simply acts as an inert electrode through which the inflow or outflow of electrons takes place.
When in a cell, this electrode acts as the anode, i.e., oxidation takes place, the following reaction takes place:
\[{{H}_{2}}\to 2{{H}^{+}}+2{{e}^{-}}\]
i.e., some hydrogen gas changes into \[{{H}^{+}}\] ions which go into the solution.
When this electrode acts as cathode, i.e., reduction takes place, the following reaction occurs:
\[2{{H}^{+}}+2{{e}^{-}}\to {{H}_{2}}\]
i.e., some \[{{H}^{+}}\] ions from the solution change into\[{{H}_{2}}\] gas. Thus, the electrode is reversible with respect to ions. This electrode is usually represented as:
\[Pt,{{H}_{2}}(g)|{{H}^{+}}(Conc=c)\]
The electrode potential of the standard hydrogen electrode is taken as 0.000 at 298 K.
The diagram of the standard hydrogen electrode is given below:
Note: The standard electrode potential of zinc was calculated with a standard electrode which was equal to 0.76 volts. The standard electrode potential of copper was calculated with a standard electrode which was equal to 0.34 volt. In common practice, all the electrode potentials are expressed as reduction potentials.
Complete answer:
The absolute value of the electrode potential of a single electrode called a single electrode potential cannot be determined because the oxidation half-reaction or reduction half-reaction cannot take place alone. It can only be measured by using some electrode as a reference electrode. The reference electrode used is the standard or normal hydrogen electrode (S.H.E or N.H.E). In the standard hydrogen gas electrode, hydrogen gas at 1 bar pressure is passed into 1 M HCl at 298 K in which a foil of platinum-coated with platinum black (finely divided platinum) remains immersed. It simply acts as an inert electrode through which the inflow or outflow of electrons takes place.
When in a cell, this electrode acts as the anode, i.e., oxidation takes place, the following reaction takes place:
\[{{H}_{2}}\to 2{{H}^{+}}+2{{e}^{-}}\]
i.e., some hydrogen gas changes into \[{{H}^{+}}\] ions which go into the solution.
When this electrode acts as cathode, i.e., reduction takes place, the following reaction occurs:
\[2{{H}^{+}}+2{{e}^{-}}\to {{H}_{2}}\]
i.e., some \[{{H}^{+}}\] ions from the solution change into\[{{H}_{2}}\] gas. Thus, the electrode is reversible with respect to ions. This electrode is usually represented as:
\[Pt,{{H}_{2}}(g)|{{H}^{+}}(Conc=c)\]
The electrode potential of the standard hydrogen electrode is taken as 0.000 at 298 K.
The diagram of the standard hydrogen electrode is given below:

Note: The standard electrode potential of zinc was calculated with a standard electrode which was equal to 0.76 volts. The standard electrode potential of copper was calculated with a standard electrode which was equal to 0.34 volt. In common practice, all the electrode potentials are expressed as reduction potentials.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




