
Define isomerism. Give IUPAC the name of an isomer of ${C_4}{H_{10}}$ which has branched chain.
Answer
569.7k+ views
Hint: Isomerism is a term that is related to the existence of two or more compounds differing in their chemical structure. Isomers are the compounds that differ in spatial arrangement of atoms in space. Hydrocarbons are the compounds that contain only carbon and hydrogen.
Complete step by step answer:
Isomerism is a phenomenon in which more than one compound has the same chemical formula but different chemical structures.
Chemical compounds that have identical chemical formulas but have different arrangements of atoms and differ in their properties are called isomers.
There are different types of isomers. Constitutional isomers are one of them.
Constitutional isomers : Isomers that differ in connectivity are called constitutional isomers. They have the same parts but those parts are connected to each other in a different manner.
The given compound is ${C_4}{H_{10}}$ . It is a hydrocarbon. Hydrocarbons are the compounds that contain only hydrogen and carbon. There are different types of hydrocarbon- saturated and unsaturated.
Saturated compounds have a single bond in the molecule. Such compounds belong to the class “alkanes”. Alkanes have a general molecular formula ${C_n}{H_{2n + 2}}$ where $n$ is the number of carbon atoms. The first member of the series is methane $(C{H_4})$ , ethane $({C_2}{H_6})$, propane $({C_3}{H_8})$ . So ${C_4}{H_{10}}$ belongs to the alkane family and is called as butane. Its structure can be shown as-
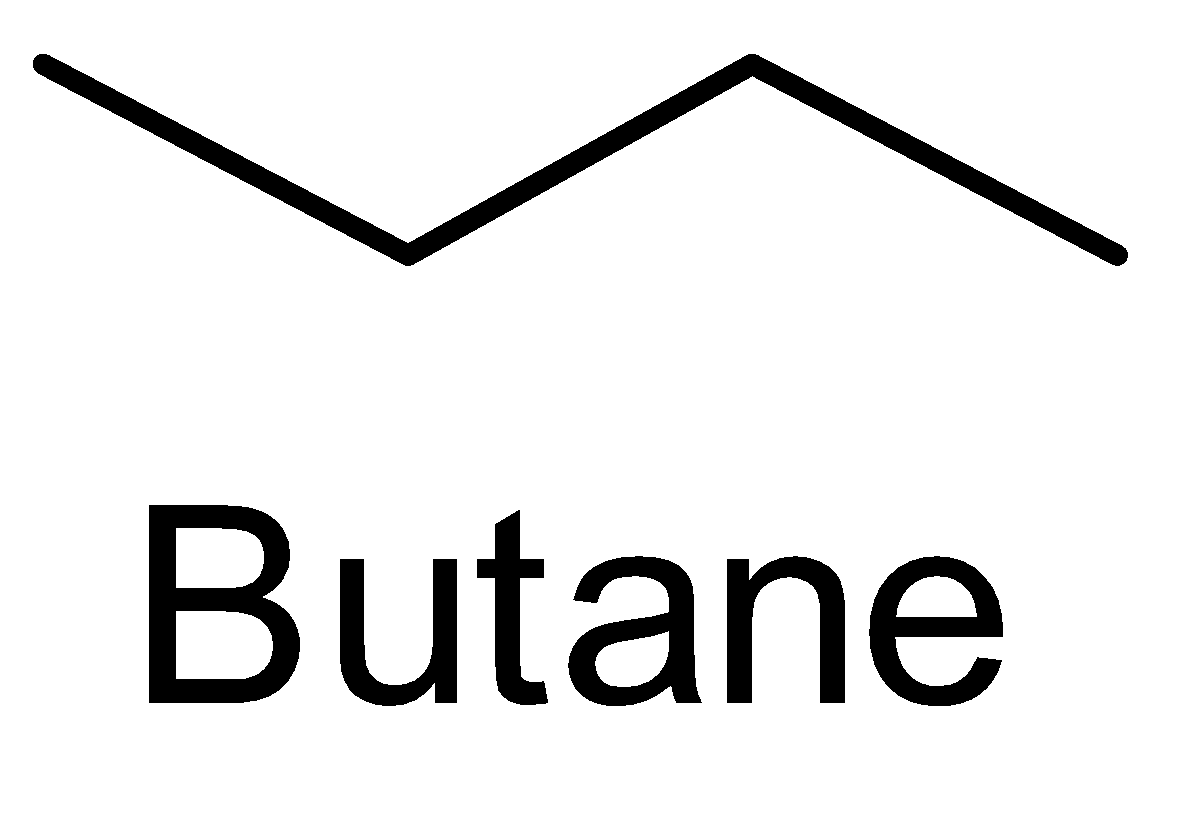
The simplest hydrocarbons- methane $(C{H_4})$ , ethane $({C_2}{H_6})$ and propane $({C_3}{H_8})$ have no constitutional isomers as there is no way to connect the carbon and hydrogen in a different way but after propane all other homologous members have constitutional isomers.
IUPAC name of isomer ${C_4}{H_{10}}$ which has branched chain is $2 - $ methyl propane. There are also other isomers possible but the isomer which has branched chain is $2 - $ methyl propane.
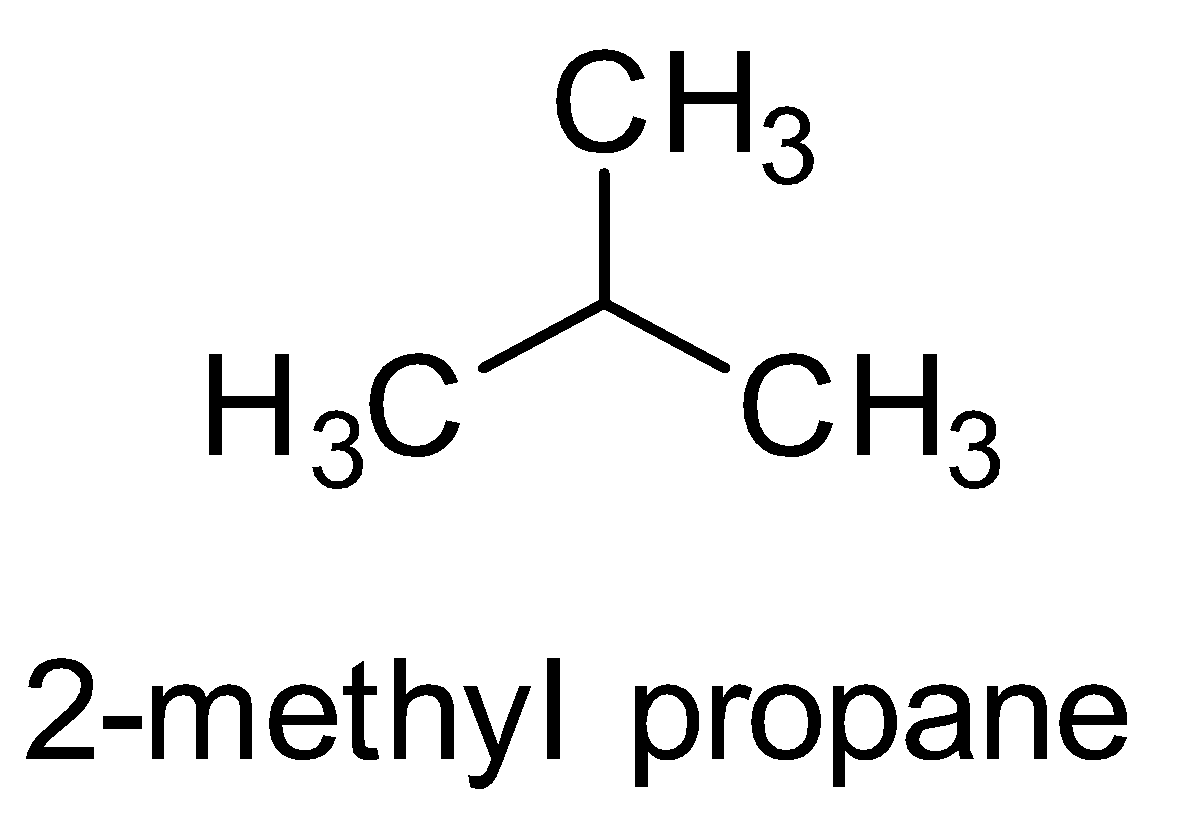
Note: The given compound was a saturated compound with more than three carbon atoms and hence was capable of showing positional isomerism. Saturated compounds can show all types of isomerism except geometrical isomerism.
Unsaturated compounds show geometrical isomerism because in unsaturated compounds the position of molecules are locked to each other due to the presence of ring structure or double bond.
Complete step by step answer:
Isomerism is a phenomenon in which more than one compound has the same chemical formula but different chemical structures.
Chemical compounds that have identical chemical formulas but have different arrangements of atoms and differ in their properties are called isomers.
There are different types of isomers. Constitutional isomers are one of them.
Constitutional isomers : Isomers that differ in connectivity are called constitutional isomers. They have the same parts but those parts are connected to each other in a different manner.
The given compound is ${C_4}{H_{10}}$ . It is a hydrocarbon. Hydrocarbons are the compounds that contain only hydrogen and carbon. There are different types of hydrocarbon- saturated and unsaturated.
Saturated compounds have a single bond in the molecule. Such compounds belong to the class “alkanes”. Alkanes have a general molecular formula ${C_n}{H_{2n + 2}}$ where $n$ is the number of carbon atoms. The first member of the series is methane $(C{H_4})$ , ethane $({C_2}{H_6})$, propane $({C_3}{H_8})$ . So ${C_4}{H_{10}}$ belongs to the alkane family and is called as butane. Its structure can be shown as-

The simplest hydrocarbons- methane $(C{H_4})$ , ethane $({C_2}{H_6})$ and propane $({C_3}{H_8})$ have no constitutional isomers as there is no way to connect the carbon and hydrogen in a different way but after propane all other homologous members have constitutional isomers.
IUPAC name of isomer ${C_4}{H_{10}}$ which has branched chain is $2 - $ methyl propane. There are also other isomers possible but the isomer which has branched chain is $2 - $ methyl propane.

Note: The given compound was a saturated compound with more than three carbon atoms and hence was capable of showing positional isomerism. Saturated compounds can show all types of isomerism except geometrical isomerism.
Unsaturated compounds show geometrical isomerism because in unsaturated compounds the position of molecules are locked to each other due to the presence of ring structure or double bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




