
Define isomerism.
Answer
566.7k+ views
Hint: Isomerism is a term that is related to the existence of two or more compounds differing in their chemical structure.
Isomers differ in structural or spatial arrangement of atoms in space.
In air pollution chemistry, they are very significant since even marginally distinct structures can elicit drastic variations in chemical and physical properties.
Complete step by step answer:
Let us first understand the concept of isomerism;
Isomerism is a phenomenon in which more than one compound has the same chemical formula but different chemical structures.
Chemical compounds that have identical chemical formulas but have different arrangements of atoms and differ in their properties are called isomers.
There are generally two types of isomers:
(a) Constitutional isomers
(b) Stereoisomers
constitutional isomers: Isomers that differ in connectivity are called constitutional isomers. They have the same parts but those parts are connected to each other in a different manner.
The simplest hydrocarbons- methane ($C{H_4}$), ethane (${C_2}{H_6}$ ) and propane ($C{H_3}C{H_2}C{H_3}$ ) have no constitutional isomers as there is no way to connect the carbon and hydrogen in a different way but after propane all other homologous members have constitutional isomers.
Stereoisomers: Isomers that have the chemical composition but differ in the orientation of atoms in space. We can further classify stereoisomerism into:
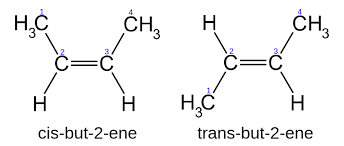
Geometrical isomerism: This type of isomerism arises in the compounds which have double bonds and have restricted rotation around double bonds. For example: cis but- 2- ene and trans but-2-ene.
Optical isomerism: This type of isomerism occurs in the compounds which contain the same kind of atoms that have the same molecular formula but have different bonding arrangements of atoms. Generally optical isomers are mirror images of each other. Only those carbon compounds are optical isomers that have non superimposable mirror images and can be found in the substances that have asymmetric carbon atoms means carbon atoms are attached to different groups. Examples of optical isomers:

Note: Only saturated compounds show chain isomerism. Unsaturated compounds show geometrical isomerism because in unsaturated compounds the positions of molecules are locked to each other due to the presence of ring structure or double bond. The geometrical isomers of unsaturated compounds in case of alkenes are cis or trans, E or Z alkenes.
Isomers differ in structural or spatial arrangement of atoms in space.
In air pollution chemistry, they are very significant since even marginally distinct structures can elicit drastic variations in chemical and physical properties.
Complete step by step answer:
Let us first understand the concept of isomerism;
Isomerism is a phenomenon in which more than one compound has the same chemical formula but different chemical structures.
Chemical compounds that have identical chemical formulas but have different arrangements of atoms and differ in their properties are called isomers.
There are generally two types of isomers:
(a) Constitutional isomers
(b) Stereoisomers
constitutional isomers: Isomers that differ in connectivity are called constitutional isomers. They have the same parts but those parts are connected to each other in a different manner.
The simplest hydrocarbons- methane ($C{H_4}$), ethane (${C_2}{H_6}$ ) and propane ($C{H_3}C{H_2}C{H_3}$ ) have no constitutional isomers as there is no way to connect the carbon and hydrogen in a different way but after propane all other homologous members have constitutional isomers.
Stereoisomers: Isomers that have the chemical composition but differ in the orientation of atoms in space. We can further classify stereoisomerism into:
Geometrical isomerism: This type of isomerism arises in the compounds which have double bonds and have restricted rotation around double bonds. For example: cis but- 2- ene and trans but-2-ene.

Optical isomerism: This type of isomerism occurs in the compounds which contain the same kind of atoms that have the same molecular formula but have different bonding arrangements of atoms. Generally optical isomers are mirror images of each other. Only those carbon compounds are optical isomers that have non superimposable mirror images and can be found in the substances that have asymmetric carbon atoms means carbon atoms are attached to different groups. Examples of optical isomers:

Note: Only saturated compounds show chain isomerism. Unsaturated compounds show geometrical isomerism because in unsaturated compounds the positions of molecules are locked to each other due to the presence of ring structure or double bond. The geometrical isomers of unsaturated compounds in case of alkenes are cis or trans, E or Z alkenes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




