
What is decreasing order of boiling point-
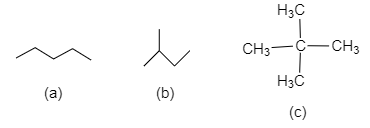
$A)$ $a > b > c$
$B)$ $b > c > a$
$C)$ $a > c > b$
$D)$ $c > b > a$

Answer
504.6k+ views
Hint: Boiling point of a substance is the temperature at which the vapour pressure of liquid is equal to the atmospheric pressure. Boiling point of an organic compound can give information about the other physical properties and structural characteristics.
Complete answer:
Boiling point of a compound tells about its volatility. The higher the boiling point, the less volatile is the compound and lower the boiling point, the more volatile is the compound.
The length of the carbon-carbon chain affects the boiling point of the compound. As the length of the carbon chain increases, the boiling point also increases. This is because the intermolecular forces between the molecules increases as the molecule gets longer. As a result, more energy will be required to overcome this force of attraction and hence the boiling point increases.
Branching in the compound decreases the boiling point. This is because the Vander Waals dispersion force is directly proportional to the surface area. Branching in the molecule will decrease the surface area as a result of which attractive forces between individual molecules decrease and hence the boiling point decreases.
Now coming to our question-
It can be seen that in (c) 2,2-dimethylpropane there are two branched methyl groups and in (b)2-methylbutane there is one branched methyl group while in (a) n-pentane there is a straight chain of five carbons. As we have seen, more branching leads to lower intermolecular forces between the molecules and hence lower boiling point.
Thus, the decreasing order of boiling point is;
$a > b > c$
So, the correct answer is “Option A”.
Note:
We have seen that boiling point depends upon the length of the carbon chain and branching. It also depends upon the polarity of the molecule. It was found that greater the polarity, higher is the boiling point.
Complete answer:
Boiling point of a compound tells about its volatility. The higher the boiling point, the less volatile is the compound and lower the boiling point, the more volatile is the compound.
The length of the carbon-carbon chain affects the boiling point of the compound. As the length of the carbon chain increases, the boiling point also increases. This is because the intermolecular forces between the molecules increases as the molecule gets longer. As a result, more energy will be required to overcome this force of attraction and hence the boiling point increases.
Branching in the compound decreases the boiling point. This is because the Vander Waals dispersion force is directly proportional to the surface area. Branching in the molecule will decrease the surface area as a result of which attractive forces between individual molecules decrease and hence the boiling point decreases.
Now coming to our question-
It can be seen that in (c) 2,2-dimethylpropane there are two branched methyl groups and in (b)2-methylbutane there is one branched methyl group while in (a) n-pentane there is a straight chain of five carbons. As we have seen, more branching leads to lower intermolecular forces between the molecules and hence lower boiling point.
Thus, the decreasing order of boiling point is;
$a > b > c$
So, the correct answer is “Option A”.
Note:
We have seen that boiling point depends upon the length of the carbon chain and branching. It also depends upon the polarity of the molecule. It was found that greater the polarity, higher is the boiling point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




