
D(+)-glucose reacts with hydroxyl amine and yields oxime. What would be the structure of the oxime?
Answer
590.1k+ views
Hint: The molecular formula of hydroxyl amine is\[N{{H}_{2}}OH\]. Generally hydroxyl amine reacts with carbonyl carbon and forms respective oxime as a product. Glucose also reacts with hydroxyl amine due to the presence of the aldehyde functional group in D(+)-glucose.
Complete step by step answer:
-The structure of D(+)-glucose is as follows.
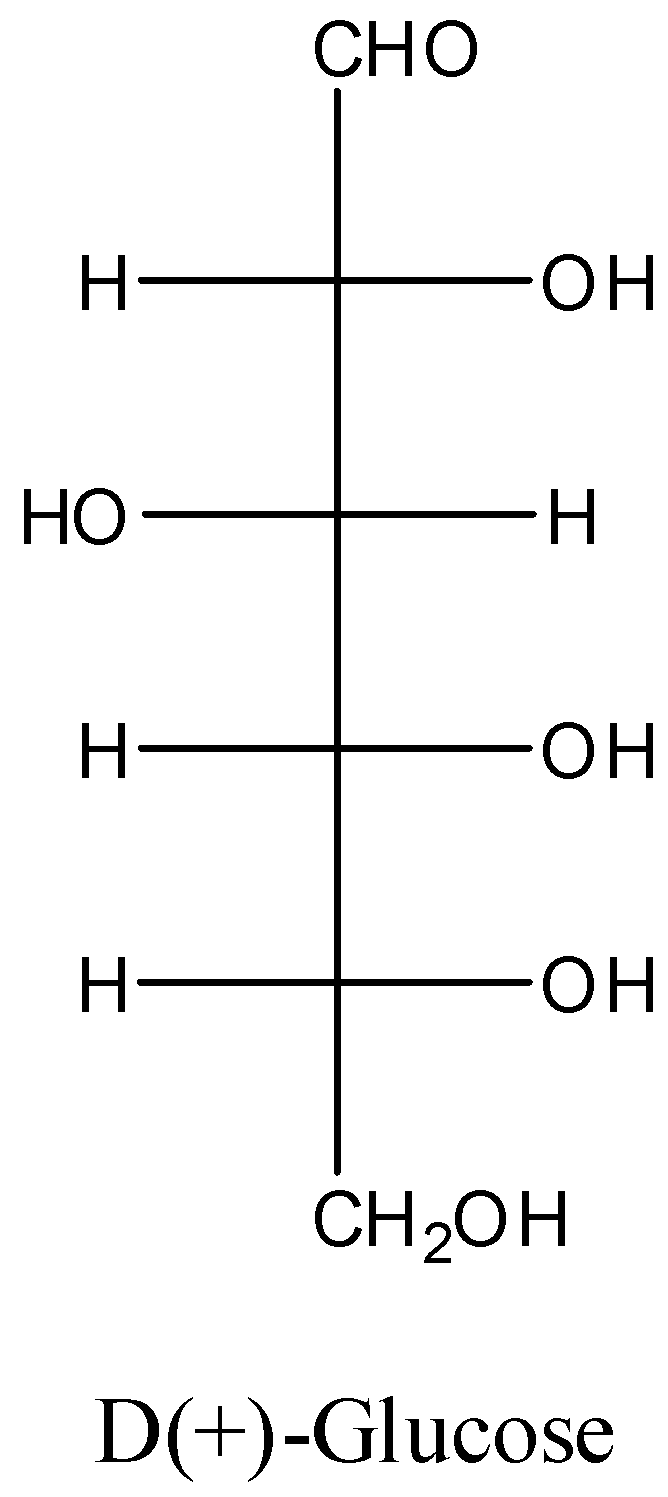
-The D(+)-glucose contains aldehyde groups in its structure.
-Then the aldehyde reacts with hydroxylamine and forms aldoxime structure.
-The chemical reaction of D(+)-glucose with hydroxylamine is as follows.
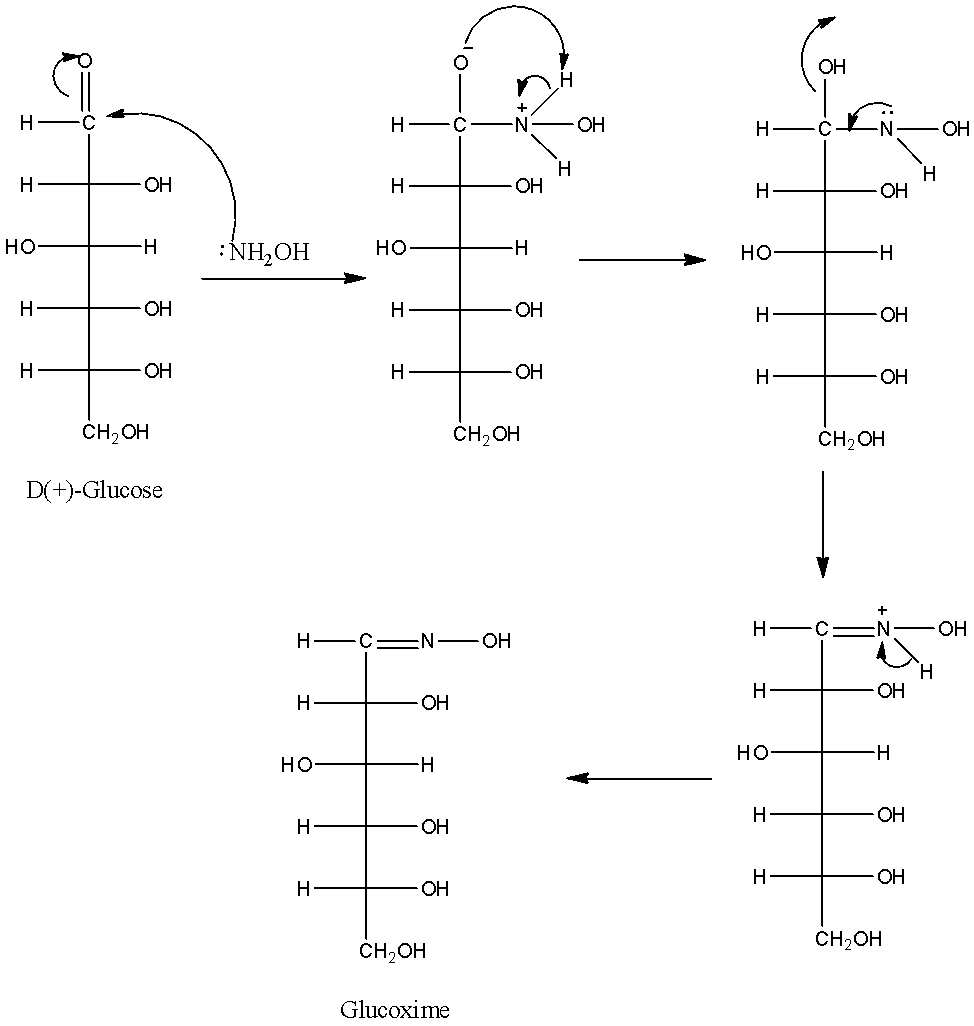
-The structure of the glucoxime is as follows.

Additional information:
-Almost maximum oximes are solids in nature and it is useful to find the presence of aldehyde or ketone functional groups in the given compound.
-Oximes are colorless crystals and less soluble in water.
-Oxime derivatives are used as medicines for nerve agents (A nerve agent deactivates acetylcholinesterase by phosphorylation reaction).
-Oximes are the chemical derivatives and belong to the category of imines.
-Dimethylglyoxime is a famous oxime used in the analysis of nickel.
-Occasionally oximes involves in multistep chemical synthesis to guard carbonyl compound
-Oximes exhibit both acidic (Weak acid) and base properties and are toxic in nature.
-Oximes are going to decompose while heating and create massive explosions.
Note: In Japan, perillaldehyde (an oxime) is used as an artificial sweetener. Methyl ethyl ketoxime is used in oil paints and as a preservative for avoiding the skin from chemicals. Acetone oxime is used to reduce the corrosion which reduces the toxicity.
Complete step by step answer:
-The structure of D(+)-glucose is as follows.

-The D(+)-glucose contains aldehyde groups in its structure.
-Then the aldehyde reacts with hydroxylamine and forms aldoxime structure.
-The chemical reaction of D(+)-glucose with hydroxylamine is as follows.

-The structure of the glucoxime is as follows.

Additional information:
-Almost maximum oximes are solids in nature and it is useful to find the presence of aldehyde or ketone functional groups in the given compound.
-Oximes are colorless crystals and less soluble in water.
-Oxime derivatives are used as medicines for nerve agents (A nerve agent deactivates acetylcholinesterase by phosphorylation reaction).
-Oximes are the chemical derivatives and belong to the category of imines.
-Dimethylglyoxime is a famous oxime used in the analysis of nickel.
-Occasionally oximes involves in multistep chemical synthesis to guard carbonyl compound
-Oximes exhibit both acidic (Weak acid) and base properties and are toxic in nature.
-Oximes are going to decompose while heating and create massive explosions.
Note: In Japan, perillaldehyde (an oxime) is used as an artificial sweetener. Methyl ethyl ketoxime is used in oil paints and as a preservative for avoiding the skin from chemicals. Acetone oxime is used to reduce the corrosion which reduces the toxicity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




