
Cyclohexanol is
A.Primary alcohol
B.Secondary alcohol
C.Tertiary alcohol
D.Phenol
Answer
502.2k+ views
Hint: We need to know that alcohols are those natural mixtures, which are portrayed by the presence of one, two or more hydroxyl gatherings $\left( { - OH} \right)$ that are joined to the carbon molecule in an alkyl gathering or hydrocarbon chain. These alcohols are viewed as the subordinates of water where one among the hydrogen iotas are supplanted by an alkyl bunch.
Complete answer:
We have to know that, alcohols are known to be one among the vast majority of the normally happening natural mixtures. These are used as sugars, planning of fragrances, and now and again during the time spent orchestrating different mixtures, wherein some others are plentifully fabricated in natural synthetic compounds coming in different businesses. Alcohols are separated depending on the presence of hydroxyl bundles. The area of this hydroxyl bunch also will change the physical and synthetic properties of any liquor.
There are three sorts of liquor. Alcohols are named essential, optional or tertiary alcohols.
Cyclohexanol is an alcohol that comprises cyclohexane bearing a solitary hydroxy substituent. The parent of the class is cyclohexanol. It has a job that is dissolvable. It is an auxiliary liquor and an individual from cyclohexanol. Therefore, the structure of cyclohexanol has to be drawn below,
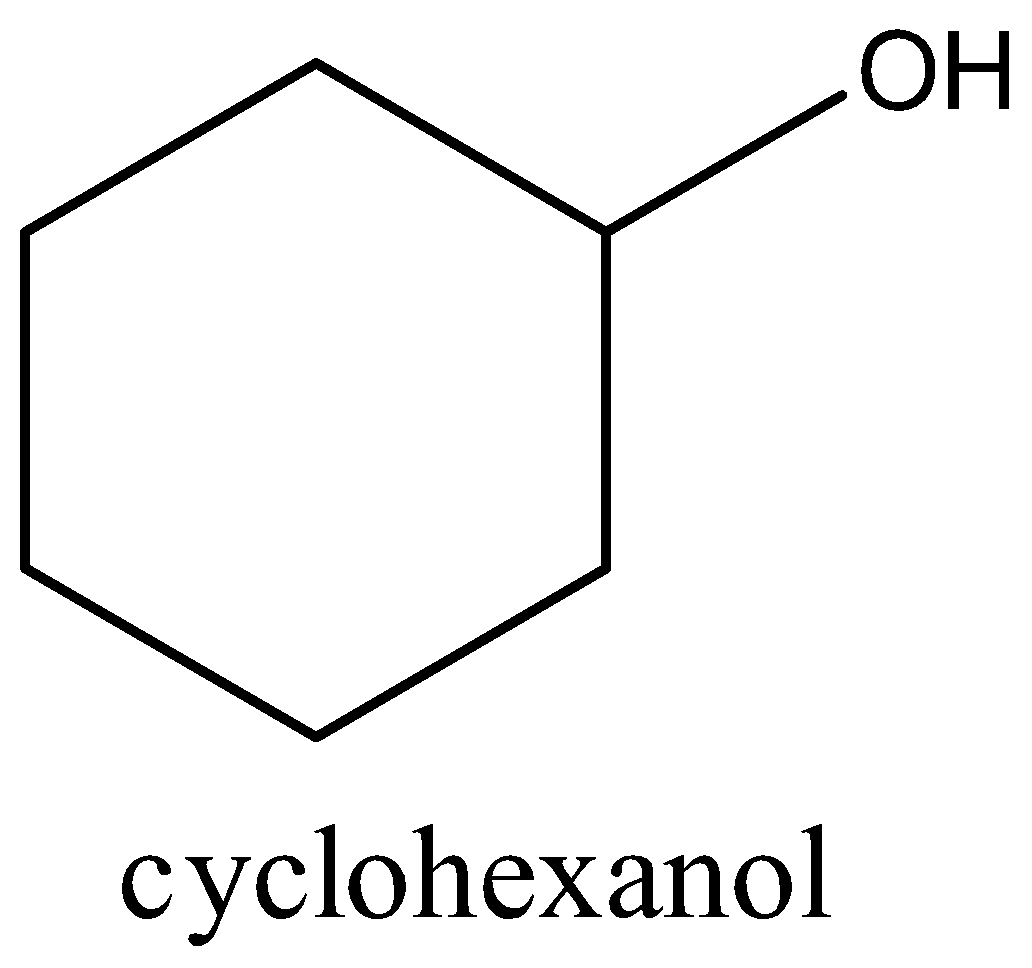
Therefore, the correct option is (B) secondary alcohol.
Note:
We have to know that liquor ethanol is utilized as a germicide agent. Some alcohols are utilized as powers in the inside ignition motors like the methanol. In the field of medication, a couple of them are utilized as additives for the examples in research centers.
Complete answer:
We have to know that, alcohols are known to be one among the vast majority of the normally happening natural mixtures. These are used as sugars, planning of fragrances, and now and again during the time spent orchestrating different mixtures, wherein some others are plentifully fabricated in natural synthetic compounds coming in different businesses. Alcohols are separated depending on the presence of hydroxyl bundles. The area of this hydroxyl bunch also will change the physical and synthetic properties of any liquor.
There are three sorts of liquor. Alcohols are named essential, optional or tertiary alcohols.
Cyclohexanol is an alcohol that comprises cyclohexane bearing a solitary hydroxy substituent. The parent of the class is cyclohexanol. It has a job that is dissolvable. It is an auxiliary liquor and an individual from cyclohexanol. Therefore, the structure of cyclohexanol has to be drawn below,

Therefore, the correct option is (B) secondary alcohol.
Note:
We have to know that liquor ethanol is utilized as a germicide agent. Some alcohols are utilized as powers in the inside ignition motors like the methanol. In the field of medication, a couple of them are utilized as additives for the examples in research centers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




