
Cyclobutene on oxidation with \[KMn{{O}_{4}}\] gives:
A. Succinic anhydride
B. Succinic acid
C. Oxalic acid
D. 1,2-dihydroxy cyclobutane.
Answer
591.9k+ views
Hint: The presence of unsaturation in organic molecules can be tested using acidified potassium permanganate (\[KMn{{O}_{4}}\]) solution. Generally alkenes will give diols with potassium permanganate. But cycloalkenes having high strain means (less than 5 carbons) will give a dicarboxylic derivative as a product.
Complete answer:
The structure of cyclobutene as follows.
The reaction of cyclobutene with potassium permanganate is as follows.
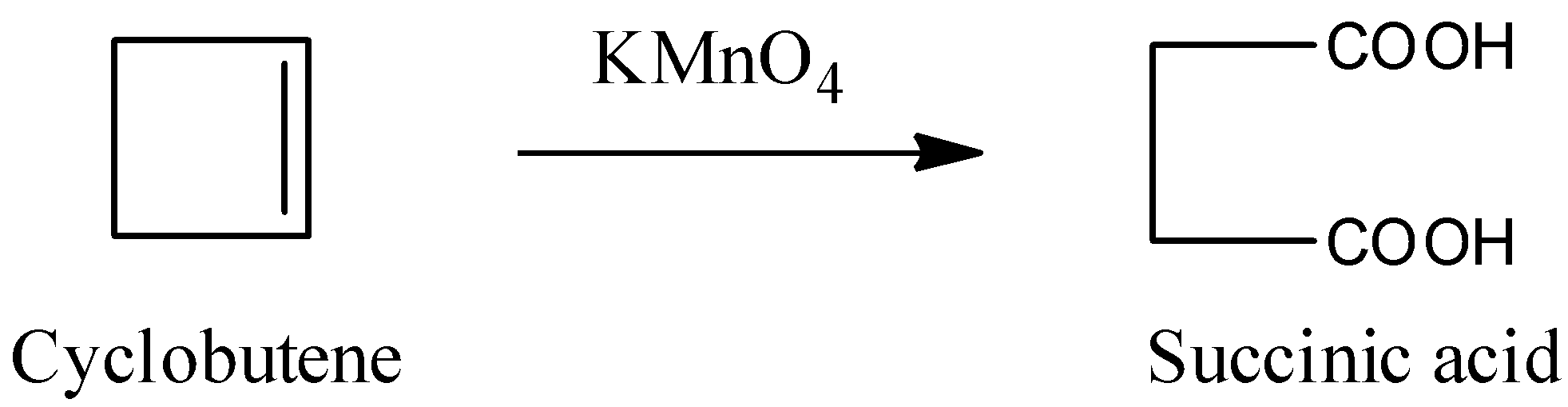
In the above reaction alkene is converted into carboxylic acid.
The product which is formed by the reaction of cyclobutene with \[KMn{{O}_{4}}\] is succinic acid.
We can use \[KMn{{O}_{4}}\] to prepare small chains of carboxylic acids.
Coming to the given options, option A, succinic anhydride. It is wrong because succinic anhydride is not going to form as a product.
Coming to option C, oxalic acid, it is also wrong oxalic acid contains two carbons in its structure. But the formed product in the above reaction contains four carbons in its structure.
Coming to option D, 1,2-dihydroxy cyclobutane. It is also wrong because in case of cyclobutene hydroxyl groups are not formed when cyclobutene reacts with potassium permanganate.
Coming to option B. Succinic acid, it is correct because succinic acid forms when cyclobutene reacts with potassium permanganate.
So, the correct answer is “Option B”.
Note:
Purple color of \[KMn{{O}_{4}}\] (Potassium Permanganate) changes colorless in the presence of unsaturation in the reactants. Also \[KMn{{O}_{4}}\] acts as an oxidizing agent in the reaction mentioned in the above solution due to the addition of oxygen atoms to the alkene.
Complete answer:
The structure of cyclobutene as follows.

The reaction of cyclobutene with potassium permanganate is as follows.

In the above reaction alkene is converted into carboxylic acid.
The product which is formed by the reaction of cyclobutene with \[KMn{{O}_{4}}\] is succinic acid.
We can use \[KMn{{O}_{4}}\] to prepare small chains of carboxylic acids.
Coming to the given options, option A, succinic anhydride. It is wrong because succinic anhydride is not going to form as a product.
Coming to option C, oxalic acid, it is also wrong oxalic acid contains two carbons in its structure. But the formed product in the above reaction contains four carbons in its structure.
Coming to option D, 1,2-dihydroxy cyclobutane. It is also wrong because in case of cyclobutene hydroxyl groups are not formed when cyclobutene reacts with potassium permanganate.
Coming to option B. Succinic acid, it is correct because succinic acid forms when cyclobutene reacts with potassium permanganate.
So, the correct answer is “Option B”.
Note:
Purple color of \[KMn{{O}_{4}}\] (Potassium Permanganate) changes colorless in the presence of unsaturation in the reactants. Also \[KMn{{O}_{4}}\] acts as an oxidizing agent in the reaction mentioned in the above solution due to the addition of oxygen atoms to the alkene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




