
Cyanohydrin of which compound on hydrolysis will give lactic acid?
A. \[{{\rm{C}}_6}{{\rm{H}}_5}{\rm{CHO}}\]
B. \[{\rm{HCHO}}\]
C. \[{\rm{C}}{{\rm{H}}_3}{\rm{CHO}}\]
D. \[{\rm{C}}{{\rm{H}}_3}{\rm{C}}{{\rm{H}}_2}{\rm{CHO}}\]
Answer
573.3k+ views
Hint: Lactic acid contains three carbon atoms. It is obtained from the cyanohydrin of an aldehyde. The number of carbon atoms in the cyanohydrin will be the same as the number of carbon atoms in lactic acid. But the starting aldehyde will have one less carbon atom, as one carbon comes from the cyano group.
Complete step by step answer:
The aldehydes react with hydrogen cyanide to form cyanohydrin.

In the above reaction, ‘R’ represents an alkyl group. The cyanohydrin has an additional carbon atom than aldehyde. This additional carbon atom comes from hydrogen cyanide. The cyano group of hydrogen cyanide is added to carbonyl carbon and the hydrogen atom of hydrogen cyanide is added to carbonyl oxygen. A new carbon-carbon single bond is formed and the carbon-oxygen double bond is converted into a single bond. Aldehyde contains carbonyl groups whereas cyanohydrin contains one hydroxyl group and one cyano group.
The hydrolysis of cyanohydrin gives alpha hydroxy, carboxylic acids.
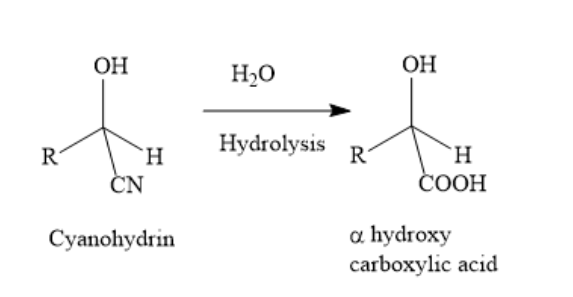
In the above reaction, the cyano group is hydrolyzed to a carboxylic acid functional group. The number of carbon atoms in the alpha hydroxy carboxylic acid is the same as the number of carbon atoms in the cyanohydrin.
When an alpha hydroxy carboxylic acid is given and it is asked to determine the structure of aldehyde, whose cyanohydrin upon hydrolysis will give the given alpha hydroxy carboxylic acid, then, decrease the number of carbon atoms of the alpha hydroxy carboxylic acid by one to obtain the number of carbon atoms of the aldehyde.
structure of lactic acid is as shown below:
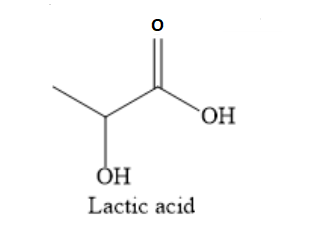
Lactic acid contains 3 carbon atoms. The corresponding aldehyde will have 3-1=2 carbon atoms.
In the given options for aldehydes, the number of carbon atoms in benzaldehyde, formaldehyde, acetaldehyde and propionaldehyde is 7,1, 2, and 3 respectively. Acetaldehyde contains 2 carbon atoms.
Hence, option C) \[{\rm{C}}{{\rm{H}}_3}{\rm{CHO}}\] is the correct answer.
Note:
Since cyano group is added to carbonyl group, cyanohydrin has one excess carbon atom than corresponding aldehyde. Hence, while determining the structure of the starting aldehyde, do not forget to account for additional carbon atoms from the cyano group.
Complete step by step answer:
The aldehydes react with hydrogen cyanide to form cyanohydrin.

In the above reaction, ‘R’ represents an alkyl group. The cyanohydrin has an additional carbon atom than aldehyde. This additional carbon atom comes from hydrogen cyanide. The cyano group of hydrogen cyanide is added to carbonyl carbon and the hydrogen atom of hydrogen cyanide is added to carbonyl oxygen. A new carbon-carbon single bond is formed and the carbon-oxygen double bond is converted into a single bond. Aldehyde contains carbonyl groups whereas cyanohydrin contains one hydroxyl group and one cyano group.
The hydrolysis of cyanohydrin gives alpha hydroxy, carboxylic acids.

In the above reaction, the cyano group is hydrolyzed to a carboxylic acid functional group. The number of carbon atoms in the alpha hydroxy carboxylic acid is the same as the number of carbon atoms in the cyanohydrin.
When an alpha hydroxy carboxylic acid is given and it is asked to determine the structure of aldehyde, whose cyanohydrin upon hydrolysis will give the given alpha hydroxy carboxylic acid, then, decrease the number of carbon atoms of the alpha hydroxy carboxylic acid by one to obtain the number of carbon atoms of the aldehyde.
structure of lactic acid is as shown below:

Lactic acid contains 3 carbon atoms. The corresponding aldehyde will have 3-1=2 carbon atoms.
In the given options for aldehydes, the number of carbon atoms in benzaldehyde, formaldehyde, acetaldehyde and propionaldehyde is 7,1, 2, and 3 respectively. Acetaldehyde contains 2 carbon atoms.
Hence, option C) \[{\rm{C}}{{\rm{H}}_3}{\rm{CHO}}\] is the correct answer.
Note:
Since cyano group is added to carbonyl group, cyanohydrin has one excess carbon atom than corresponding aldehyde. Hence, while determining the structure of the starting aldehyde, do not forget to account for additional carbon atoms from the cyano group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




