
What is cumene? How will you convert it to phenol?
Answer
497.7k+ views
Hint: We know that isopropyl benzene is known as cumene. Cumene is its common name. Most of the worldwide phenol and acetone are produced by the cumene process which is also known as Hock. This is the economical reaction that converts two very cheap starting materials into the most valuable product phenol, acetone.
Complete Step By Step Answer:
To solve this problem first, we will collect information about cumene. Cumene is the common name of the organic compound isopropyl benzene.
Now we will discuss that how isopropylbenzene/cumene is prepared in the lab-
Cumene preparation-
For the preparation of cumene, we take starting material benzene and propylene, and the reaction proceeds via Friedel crafts alkylation reaction in the presence of Lewis acid. Phosphoric acid is used as catalytic Lewis acid. This reaction takes place at high pressure and high temperature
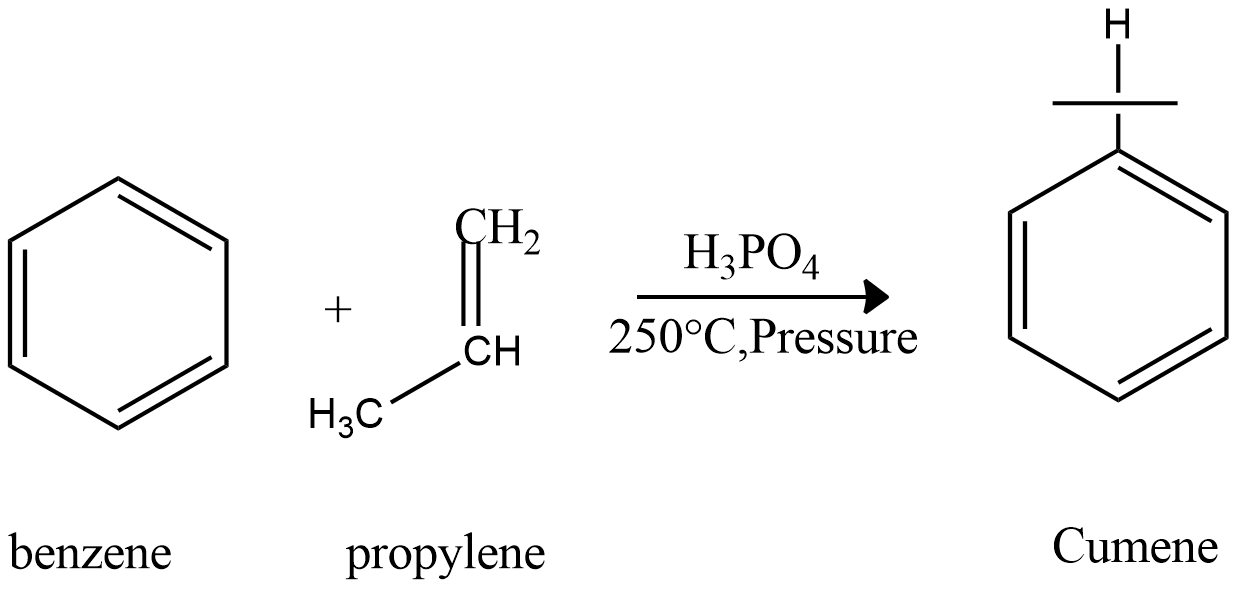
Now we will see that how phenol and by-product acetone is prepared from cumene
Step 1:
Cumene is oxidized in air to form cumene radical.
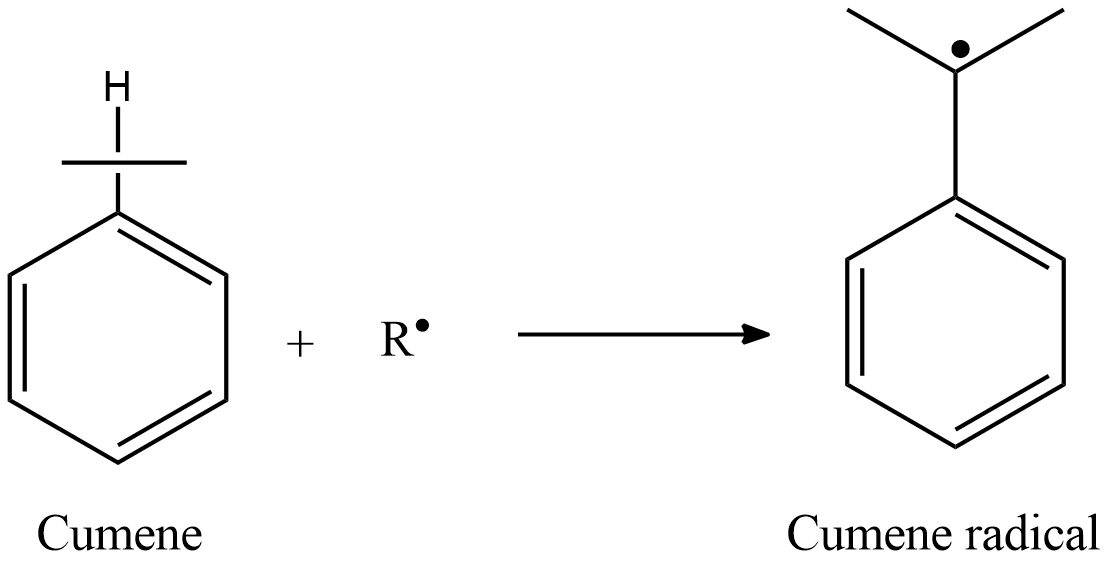
Here R represents the alkyl radical. Now we will see step 2, where this cumene radical reacts with $ {O_2} $ .
Step 2:
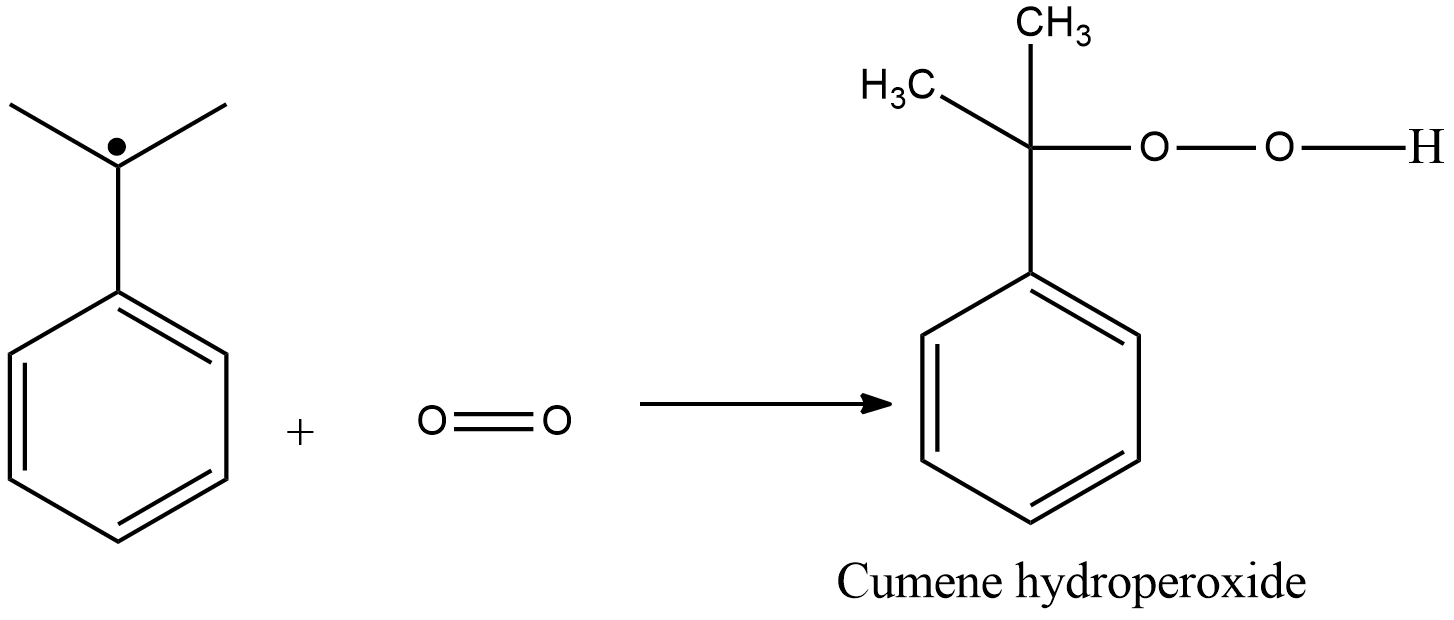
Reaction with $ {O_2} $ cumene radical produces cumene hydroperoxide. In the next step, cumene hydroperoxide converts into phenol on treatment with dilute acid. Acetone is produced as a by-product.
Step 3:
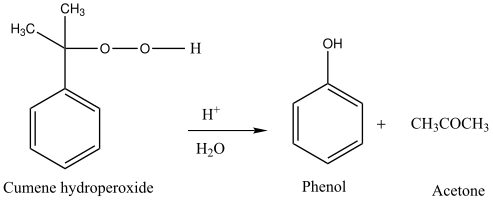
This is how cumene converts into phenol. Acetone is a by-product in this reaction but is obtained in a high amount.
Note:
In Friedel craft alkylation reaction of benzene and propylene phosphoric acid is used as catalytic lewis acid instead of aluminum halides. This process is very economical to produce phenol if there is enough demand for acetone which is formed in this reaction as a by-product in large amounts.
Complete Step By Step Answer:
To solve this problem first, we will collect information about cumene. Cumene is the common name of the organic compound isopropyl benzene.
Now we will discuss that how isopropylbenzene/cumene is prepared in the lab-
Cumene preparation-
For the preparation of cumene, we take starting material benzene and propylene, and the reaction proceeds via Friedel crafts alkylation reaction in the presence of Lewis acid. Phosphoric acid is used as catalytic Lewis acid. This reaction takes place at high pressure and high temperature

Now we will see that how phenol and by-product acetone is prepared from cumene
Step 1:
Cumene is oxidized in air to form cumene radical.

Here R represents the alkyl radical. Now we will see step 2, where this cumene radical reacts with $ {O_2} $ .
Step 2:

Reaction with $ {O_2} $ cumene radical produces cumene hydroperoxide. In the next step, cumene hydroperoxide converts into phenol on treatment with dilute acid. Acetone is produced as a by-product.
Step 3:

This is how cumene converts into phenol. Acetone is a by-product in this reaction but is obtained in a high amount.
Note:
In Friedel craft alkylation reaction of benzene and propylene phosphoric acid is used as catalytic lewis acid instead of aluminum halides. This process is very economical to produce phenol if there is enough demand for acetone which is formed in this reaction as a by-product in large amounts.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




