
Crystal field stabilization energy for the high spin ${{d}^{4}}$ octahedral complex is:
(a)- $-1.8\text{ }{{\Delta }_{\circ }}$
(b)- $-1.6\text{ }{{\Delta }_{\circ }}+P$
(c)- $-1.2\text{ }{{\Delta }_{\circ }}$
(d)- $-0.6\text{ }{{\Delta }_{\circ }}$
Answer
546.3k+ views
Hint: In octahedral complexes, there is the involvement of d-orbitals, and there are five orbitals of d. When the ligands approach the metal ion, the d-orbitals will split into two parts: ${{t}_{2}}g$ and $eg$. For high spin complexes, the pairing of the electron will not occur.
Complete answer:
The octahedral complexes are formed in the element in which there is the presence of d-orbital or we can say that in octahedral complexes, there is the involvement of d-orbitals, and there are five orbitals of d.
The names of five d-orbitals are ${{d}_{xy}},{{d}_{yz}},{{d}_{zx}},{{d}_{{{x}^{2}}-{{y}^{2}}}},\text{ and }{{d}_{{{z}^{2}}}}$.
All the energy of the five orbitals are the same but when the ligands approach the metal ion, the d-orbitals of the metal ion will split into two sets: ${{t}_{2}}g$ and $eg$. The ${{t}_{2}}g$ part contains the three orbitals and the $eg$part contains the two orbitals. The value of energy of the ${{t}_{2}}g$ orbitals is $-0.4\text{ }{{\Delta }_{\circ }}$ and the value of energy of the eg orbitals is $\text{+0}\text{.6 }{{\Delta }_{\circ }}$.
In ${{d}^{4}}$ complexes, there will be 4 electrons in the d-orbital. In the question, the complex is high spin which means that the pairing of electrons will not occur until all the orbitals will get one electron.
For filling of 4 electrons, the three ${{t}_{2}}g$ orbitals will get 3 electrons and one electron will enter the e.g. orbital.
The diagram is given below:
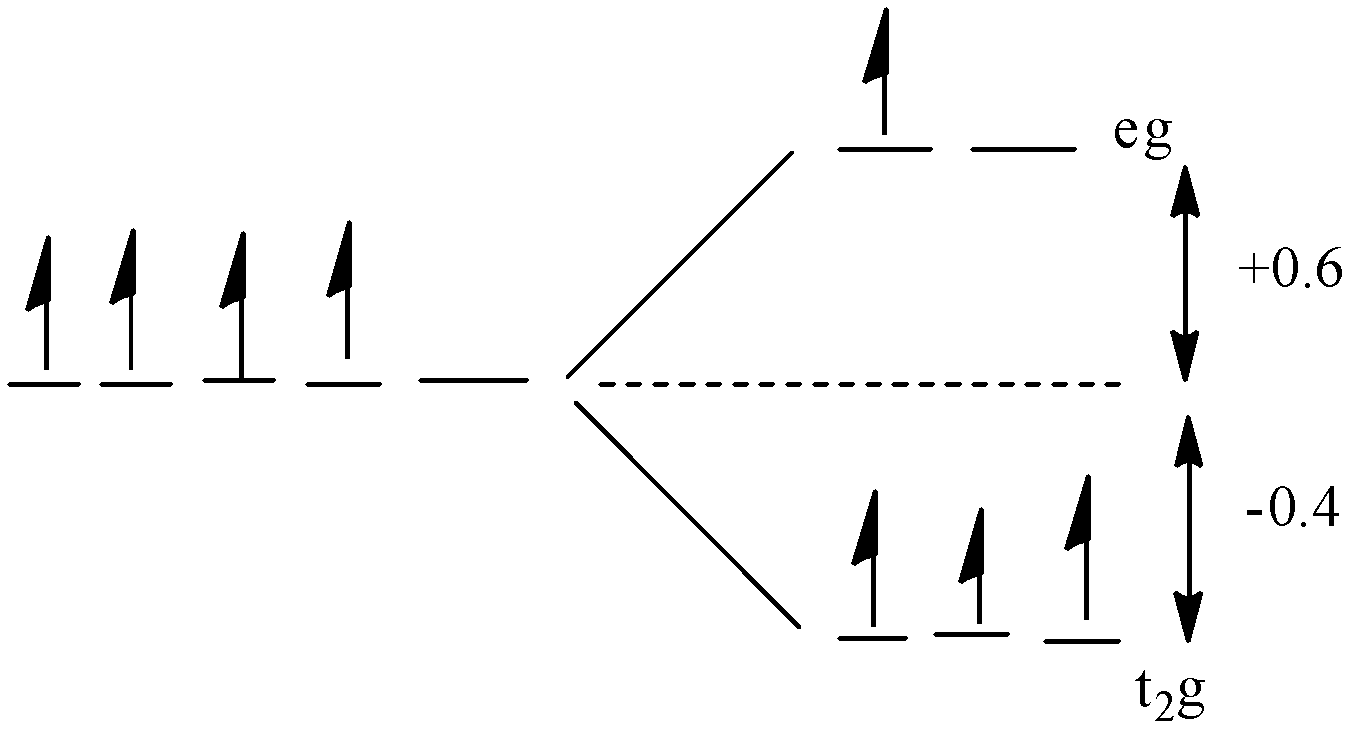
The energy will be:
$CFSE=(3\text{ x -0}\text{.4)+(1 x 0}\text{.6)}$
$CFSE=(-1.2+0.6)$
$CFSE=-0.6\text{ }{{\Delta }_{\circ }}$
So, the CFSE (Crystal field stabilization energy) for the high spin ${{d}^{4}}$ octahedral complex will be $-0.6\text{ }{{\Delta }_{\circ }}$.
Therefore, the correct answer is an option (d).
Note:
It must be noted that when the ligand is high spin complex then the pairing of the electron will occur and when the ligand is low spin complex then first the pairing of an electron in ${{t}_{2}}g$ set will occur then the electron will move into the eg orbital.
Complete answer:
The octahedral complexes are formed in the element in which there is the presence of d-orbital or we can say that in octahedral complexes, there is the involvement of d-orbitals, and there are five orbitals of d.
The names of five d-orbitals are ${{d}_{xy}},{{d}_{yz}},{{d}_{zx}},{{d}_{{{x}^{2}}-{{y}^{2}}}},\text{ and }{{d}_{{{z}^{2}}}}$.
All the energy of the five orbitals are the same but when the ligands approach the metal ion, the d-orbitals of the metal ion will split into two sets: ${{t}_{2}}g$ and $eg$. The ${{t}_{2}}g$ part contains the three orbitals and the $eg$part contains the two orbitals. The value of energy of the ${{t}_{2}}g$ orbitals is $-0.4\text{ }{{\Delta }_{\circ }}$ and the value of energy of the eg orbitals is $\text{+0}\text{.6 }{{\Delta }_{\circ }}$.
In ${{d}^{4}}$ complexes, there will be 4 electrons in the d-orbital. In the question, the complex is high spin which means that the pairing of electrons will not occur until all the orbitals will get one electron.
For filling of 4 electrons, the three ${{t}_{2}}g$ orbitals will get 3 electrons and one electron will enter the e.g. orbital.
The diagram is given below:

The energy will be:
$CFSE=(3\text{ x -0}\text{.4)+(1 x 0}\text{.6)}$
$CFSE=(-1.2+0.6)$
$CFSE=-0.6\text{ }{{\Delta }_{\circ }}$
So, the CFSE (Crystal field stabilization energy) for the high spin ${{d}^{4}}$ octahedral complex will be $-0.6\text{ }{{\Delta }_{\circ }}$.
Therefore, the correct answer is an option (d).
Note:
It must be noted that when the ligand is high spin complex then the pairing of the electron will occur and when the ligand is low spin complex then first the pairing of an electron in ${{t}_{2}}g$ set will occur then the electron will move into the eg orbital.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




