
What is the covalency of a Phosphorus atom in an excited state?
A.3
B.4
C.5
D.1
Answer
583.2k+ views
Hint: Phosphorous is a Group 15 element. Covalency of an atom is the number of pairs of electrons that can share.
Complete step by step answer:
We know that Nitrogen exhibits a maximum of Covalency of 4. In the ammonium ion Nitrogen forms 4 Covalent bonds. Also due to the non-availability of d orbitals Nitrogen can’t form pentavalent compounds.
In phosphorous d orbitals are available. They can form dπ-dπ bonds with other elements. Phosphorous. By counting the number of bonds formed by the element we can calculate the covalency of that element.
An Oxoacid is an acid containing Oxygen and at least one other element. Phosphoric acid(H3PO4) is an oxoacid of Phosphorus. In this compound Phosphorus form five Covalent bonds.
H3PO4 has a tetrahedral structure. In this Phosphorus is in +5 oxidation state. Phosphoric acid contains 3 ionizable hydrogens. All the three Hydrogens are attached to an Oxygen atom. So, we can say that the basicity of this acid is 3. i.e it is a tribasic acid. Other Oxoacids of Phosphorus include Phosphinic acid, Phosphonic acid, Phosphoric acid etc.
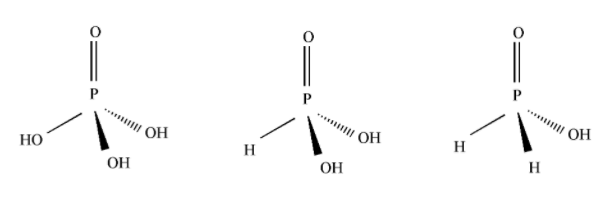
\[{H_3}P{O_4}\] (Phosphoric acid) \[{H_3}P{O_3}\] (Phosphonic acid) \[{H_3}P{O_2}\] (Phosphinic acid)
Hence, the Correct answer is Option (C) i.e 5.
Note: In the oxoacids of Phosphorus, only the Hydrogen atoms on the OH groups are ionizable. And they give \[{H^ + }\] . Hydrogen atoms attached directly to P, are not ionizable. Basicity of Phosphinic acid is 1 and Basicity of Phosphonic acid is 2.
Complete step by step answer:
We know that Nitrogen exhibits a maximum of Covalency of 4. In the ammonium ion Nitrogen forms 4 Covalent bonds. Also due to the non-availability of d orbitals Nitrogen can’t form pentavalent compounds.
In phosphorous d orbitals are available. They can form dπ-dπ bonds with other elements. Phosphorous. By counting the number of bonds formed by the element we can calculate the covalency of that element.
An Oxoacid is an acid containing Oxygen and at least one other element. Phosphoric acid(H3PO4) is an oxoacid of Phosphorus. In this compound Phosphorus form five Covalent bonds.
H3PO4 has a tetrahedral structure. In this Phosphorus is in +5 oxidation state. Phosphoric acid contains 3 ionizable hydrogens. All the three Hydrogens are attached to an Oxygen atom. So, we can say that the basicity of this acid is 3. i.e it is a tribasic acid. Other Oxoacids of Phosphorus include Phosphinic acid, Phosphonic acid, Phosphoric acid etc.

\[{H_3}P{O_4}\] (Phosphoric acid) \[{H_3}P{O_3}\] (Phosphonic acid) \[{H_3}P{O_2}\] (Phosphinic acid)
Hence, the Correct answer is Option (C) i.e 5.
Note: In the oxoacids of Phosphorus, only the Hydrogen atoms on the OH groups are ionizable. And they give \[{H^ + }\] . Hydrogen atoms attached directly to P, are not ionizable. Basicity of Phosphinic acid is 1 and Basicity of Phosphonic acid is 2.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




