
Coupling of benzene diazonium chloride with 1-naphthol in alkaline medium will give:
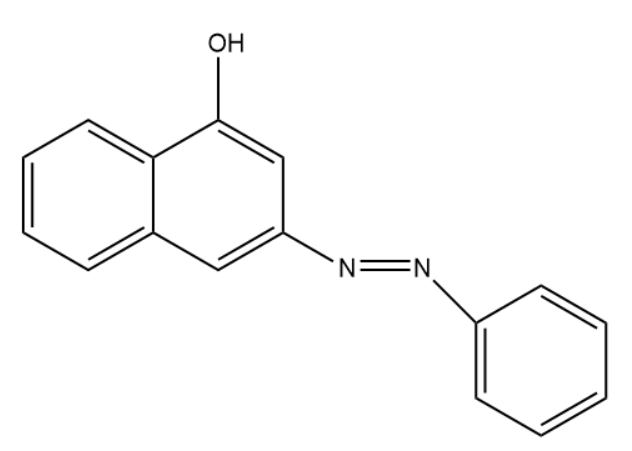
(A)
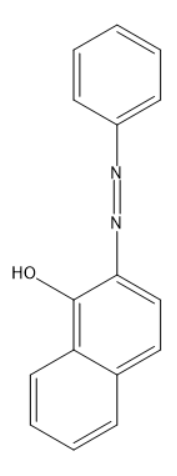
(B)
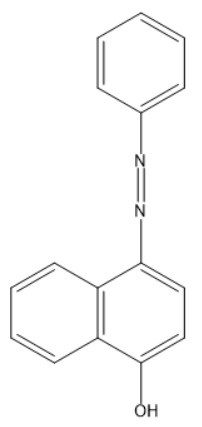
C)
D)
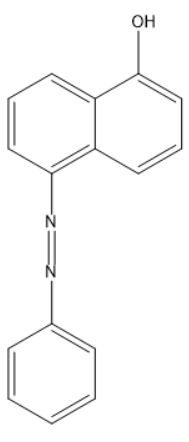




Answer
577.2k+ views
Hint: It is a type of azo coupling. In such reactions diazonium salt shows electrophilic attack on other aromatic compounds and substitution reaction occurs.
Complete Solution :
Azo coupling: Those reactions in which a diazonium compound reacts with another aromatic compound. The product of such reactions is an azo compound. These reactions come under the category of electrophilic aromatic substitution reaction, in which aryl diazonium cation behaves as electrophile and attack on activated arene which is a nucleophile.
- The given question is asking about the product of reaction between benzene diazonium chloride and 1-naphthol (alpha naphthol). So, the Product is this reaction is a dye organol brown. In this dye the electrophile aryl diazonium cation substitutes the hydrogen of at para position to the hydroxyl group of alpha naphthol among the three open positions i.e. ortho, meta, and para to the hydroxyl group.
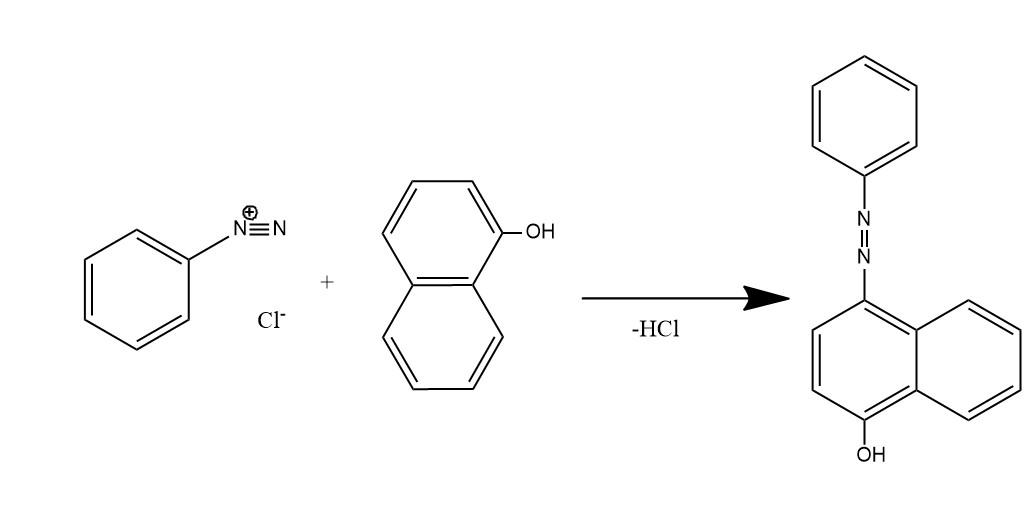
Substitution occurs at para position of naphthol ring because of two reasons:
(1) Hydroxyl groups of phenols are ortho and para directing for electrophilic substitution reactions.
(2) In naphthalene system electrophilic substitution is kinetically favored next to the other ring.
So, among ortho and para positions, para one is next to the ring which favors the substitution at para position.
So, the correct answer is “Option C”.
Note: Organol brown is also called hex type brown, a Chemical name is 4-(Phenylazo)-1-naphthol, Chemical formula is ${{C}_{16}}{{H}_{12}}{{N}_{2}}O$.
Complete Solution :
Azo coupling: Those reactions in which a diazonium compound reacts with another aromatic compound. The product of such reactions is an azo compound. These reactions come under the category of electrophilic aromatic substitution reaction, in which aryl diazonium cation behaves as electrophile and attack on activated arene which is a nucleophile.
- The given question is asking about the product of reaction between benzene diazonium chloride and 1-naphthol (alpha naphthol). So, the Product is this reaction is a dye organol brown. In this dye the electrophile aryl diazonium cation substitutes the hydrogen of at para position to the hydroxyl group of alpha naphthol among the three open positions i.e. ortho, meta, and para to the hydroxyl group.

Substitution occurs at para position of naphthol ring because of two reasons:
(1) Hydroxyl groups of phenols are ortho and para directing for electrophilic substitution reactions.
(2) In naphthalene system electrophilic substitution is kinetically favored next to the other ring.
So, among ortho and para positions, para one is next to the ring which favors the substitution at para position.

So, the correct answer is “Option C”.
Note: Organol brown is also called hex type brown, a Chemical name is 4-(Phenylazo)-1-naphthol, Chemical formula is ${{C}_{16}}{{H}_{12}}{{N}_{2}}O$.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE

Explain sex determination in humans with line diag class 12 biology CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE




