
What is the correct name of ${\rm{FeO}}$?
A. Iron (I) oxide
B. Iron oxide
C. Iron (II) oxide
D. Iron monoxide
Answer
582.3k+ views
Hint: We know that, name of a chemical compound is given using the name and oxidation state of the cation and anion of the compound. Iron is a metal and it has exhibited two oxidation states namely +2 and +3.
Complete step by step answer: First we understand how to write the chemical formula of a compound. To write the chemical formula of a compound, first we have to write the chemical symbols of the elements along with the valency then, we have to crossover the valencies of the combining elements. For example, formula is written from aluminium oxide.
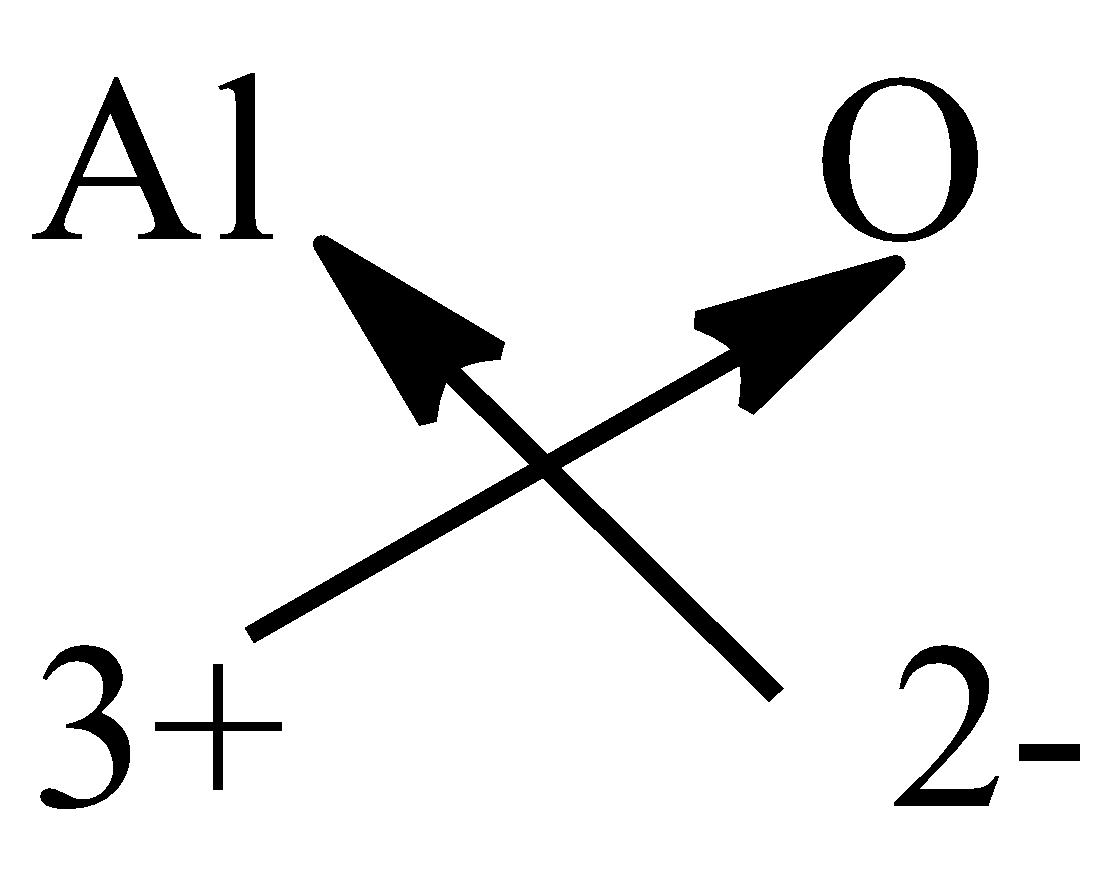
So, the formula of aluminium oxide is ${\rm{A}}{{\rm{l}}_2}{{\rm{O}}_{\rm{3}}}$
Let’s identify the correct answer from the options.
Option A says the name of FeO is Iron (I) oxide .The name Iron (I) oxide indicates that valency of iron is +1 and we know valency of oxide is -2. So, the formula is${\rm{F}}{{\rm{e}}_2}{\rm{O}}$. So, A is incorrect.
Option B says the name of FeO is iron oxide. We know another name of iron oxide is Iron (III) oxide. This indicates that, valency of iron is 3+ and that of oxide is 2-.So, the chemical formula of iron oxide is ${\rm{F}}{{\rm{e}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}$. So, option B is incorrect.
Option C says the iron name of FeO is iron (II) oxide. But, Iron (II) oxide indicates, the valency of iron is 2+ and that of oxygen is 2-. So, the formula for Iron (II) oxide is FeO.
Hence, option C is the correct answer.
Note: In naming of iron oxide, always remember that, if the iron atom possesses +2 oxidation state, the oxide is ferrous oxide $\left( {{\rm{FeO}}} \right)$ and if the iron atom possesses +3 oxidation state, the name of the iron oxide is ferric oxide $\left( {{\rm{F}}{{\rm{e}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}} \right)$.
Complete step by step answer: First we understand how to write the chemical formula of a compound. To write the chemical formula of a compound, first we have to write the chemical symbols of the elements along with the valency then, we have to crossover the valencies of the combining elements. For example, formula is written from aluminium oxide.

So, the formula of aluminium oxide is ${\rm{A}}{{\rm{l}}_2}{{\rm{O}}_{\rm{3}}}$
Let’s identify the correct answer from the options.
Option A says the name of FeO is Iron (I) oxide .The name Iron (I) oxide indicates that valency of iron is +1 and we know valency of oxide is -2. So, the formula is${\rm{F}}{{\rm{e}}_2}{\rm{O}}$. So, A is incorrect.
Option B says the name of FeO is iron oxide. We know another name of iron oxide is Iron (III) oxide. This indicates that, valency of iron is 3+ and that of oxide is 2-.So, the chemical formula of iron oxide is ${\rm{F}}{{\rm{e}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}$. So, option B is incorrect.
Option C says the iron name of FeO is iron (II) oxide. But, Iron (II) oxide indicates, the valency of iron is 2+ and that of oxygen is 2-. So, the formula for Iron (II) oxide is FeO.
Hence, option C is the correct answer.
Note: In naming of iron oxide, always remember that, if the iron atom possesses +2 oxidation state, the oxide is ferrous oxide $\left( {{\rm{FeO}}} \right)$ and if the iron atom possesses +3 oxidation state, the name of the iron oxide is ferric oxide $\left( {{\rm{F}}{{\rm{e}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}} \right)$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




