
Correct increasing order of the wavelength of absorption in the visible region for the complexes of $C{{o}^{3+}}$ is?
(A)- ${{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}$
(B)- ${{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}$
(C)- ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}$
(D)- ${{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(CN)}_{6}}]}^{3-}}$
Answer
578.7k+ views
Hint: We can calculate the order of absorption of wavelength with the help of the spectrochemical series. The spectrochemical series tells the order of the ability of the ligand to cause the splitting of the orbitals.
Complete step by step solution:
We can calculate the order of absorption of wavelength with the help of the spectrochemical series. The spectrochemical series tells the order of the ability of the ligand to cause the splitting of the orbitals. When the absorption of the wavelength in the visible region takes place the complexes are colored. When the ligand approaches the central metal atom, it causes the splitting of the d-orbitals.
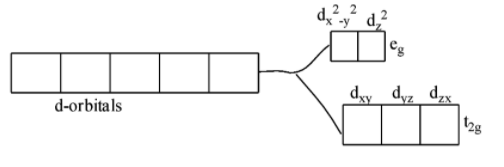
when the anion or the ligands approach the transition metal ions, their d-orbitals do not remain degenerated. They split into two sets, one consisting of lower energy orbitals (\[{{\text{t}}_{\text{2g}}}\text{which includes }{{\text{d}}_{\text{xy}}}\text{,}{{\text{d}}_{\text{yz}}}\text{,and }{{\text{d}}_{\text{zx}}}\]) and the other consisting of higher energy orbitals (\[{{e}_{\text{g}}}\text{which includes }{{\text{d}}_{{{\text{x}}^{2}}-{{y}^{2}}}}\text{,and }{{\text{d}}_{{{\text{z}}^{2}}}}\]). This is called crystal field splitting.
Thus, the electron can jump from lower energy d-orbital to higher energy d-orbital. The required amount of energy to do this is obtained by absorption of light of a particular wavelength in the region of visible light.
So as the ligands become strong field ligands, they cause more splitting and the electrons cannot jump the gap so the energy will be low.
So the decreasing order of the ligands in the spectrochemical series is $C{{N}^{-}}$ > $N{{H}_{3}}$ > ${{H}_{2}}O$ > $C{{l}^{-}}$. Therefore, the order of the absorption of the wavelength in the visible region will be reversed. So the order will:
${{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}$
Therefore, the correct answer is option (a)- ${{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}$.
Note: Crystal field splitting energy also known as crystal field stabilization energy (CFSE) tells the energy difference between the orbitals of the d-orbital. Stronger field ligands have large CFSE.
Complete step by step solution:
We can calculate the order of absorption of wavelength with the help of the spectrochemical series. The spectrochemical series tells the order of the ability of the ligand to cause the splitting of the orbitals. When the absorption of the wavelength in the visible region takes place the complexes are colored. When the ligand approaches the central metal atom, it causes the splitting of the d-orbitals.
when the anion or the ligands approach the transition metal ions, their d-orbitals do not remain degenerated. They split into two sets, one consisting of lower energy orbitals (\[{{\text{t}}_{\text{2g}}}\text{which includes }{{\text{d}}_{\text{xy}}}\text{,}{{\text{d}}_{\text{yz}}}\text{,and }{{\text{d}}_{\text{zx}}}\]) and the other consisting of higher energy orbitals (\[{{e}_{\text{g}}}\text{which includes }{{\text{d}}_{{{\text{x}}^{2}}-{{y}^{2}}}}\text{,and }{{\text{d}}_{{{\text{z}}^{2}}}}\]). This is called crystal field splitting.

Thus, the electron can jump from lower energy d-orbital to higher energy d-orbital. The required amount of energy to do this is obtained by absorption of light of a particular wavelength in the region of visible light.
So as the ligands become strong field ligands, they cause more splitting and the electrons cannot jump the gap so the energy will be low.
So the decreasing order of the ligands in the spectrochemical series is $C{{N}^{-}}$ > $N{{H}_{3}}$ > ${{H}_{2}}O$ > $C{{l}^{-}}$. Therefore, the order of the absorption of the wavelength in the visible region will be reversed. So the order will:
${{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}$
Therefore, the correct answer is option (a)- ${{[Co{{(CN)}_{6}}]}^{3-}},{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}({{H}_{2}}O)]}^{3+}},{{[Co{{(N{{H}_{3}})}_{5}}Cl]}^{2+}}$.
Note: Crystal field splitting energy also known as crystal field stabilization energy (CFSE) tells the energy difference between the orbitals of the d-orbital. Stronger field ligands have large CFSE.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




