
Coordination number of \[{\text{Cr}}\] is six. A complex with \[{{\text{C}}_{\text{2}}}{\text{O}}_{\text{4}}^{{\text{2 - }}}\], en and superoxide \[{\text{O}}_{\text{2}}^{\text{ - }}\] will be in the ratio to make complex ${{\text{[Cr(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{x}}}{{\text{(en)}}_{\text{y}}}{{\text{(}}{{\text{O}}_{\text{2}}}{\text{)}}_{\text{z}}}{\text{]}}^{\text{ - }}}$
1. \[{\text{x = 1, y = 1, z = 1}}\]
2. \[{\text{x = 1, y = 1, z = 2}}\]
3. \[{\text{x = 1, y = 2, z = 2}}\]
4. \[{\text{x = 2, y = 1, z = 1}}\]
Answer
577.5k+ views
Hint:The coordination number of chromium is six, hence it is in \[{\text{ + 3}}\] oxidation state. It can form a coordinate bond through six donor ligand atoms. Therefore, the denticity of each ligand given should be known.
Complete step by step answer:
The given coordination compounds are as follows.
${{\text{[Cr(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{x}}}{{\text{(en)}}_{\text{y}}}{{\text{(}}{{\text{O}}_{\text{2}}}{\text{)}}_{\text{z}}}{\text{]}}^{\text{ - }}}$
In this compound we have to find the \[{\text{x,y and z}}\] values.
The central metal atom – Chromium.
Chromium atomic number – \[{\text{24}}\]
Electronic configuration -\[{\text{[Ar]3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{2}}}\]. And it is ${\text{3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{2}}}$system. Hence, it has a total six valence electrons available for the formation of coordinate bonds.
For \[{\text{x}}\] value:
The ligand oxalate (${{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$) is a bi-dendate ligand means which donate two pairs of electrons to a central metal atom.
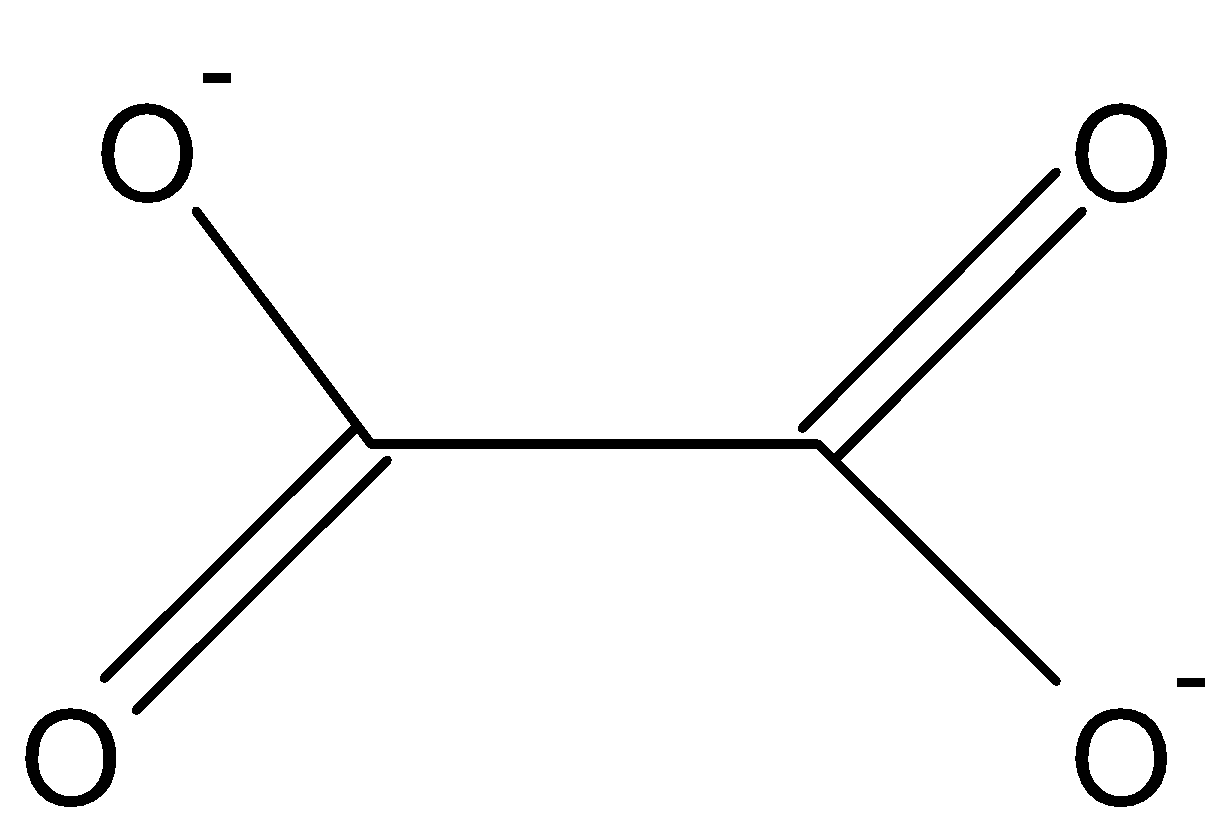
Figure 1: Oxalate ion
Hence, the coordination number is \[{\text{2}}\].
For \[{\text{y}}\] value:
The ligand ethylenediamine (en) is also bi-dendate ligand and it is a neutral ligand.
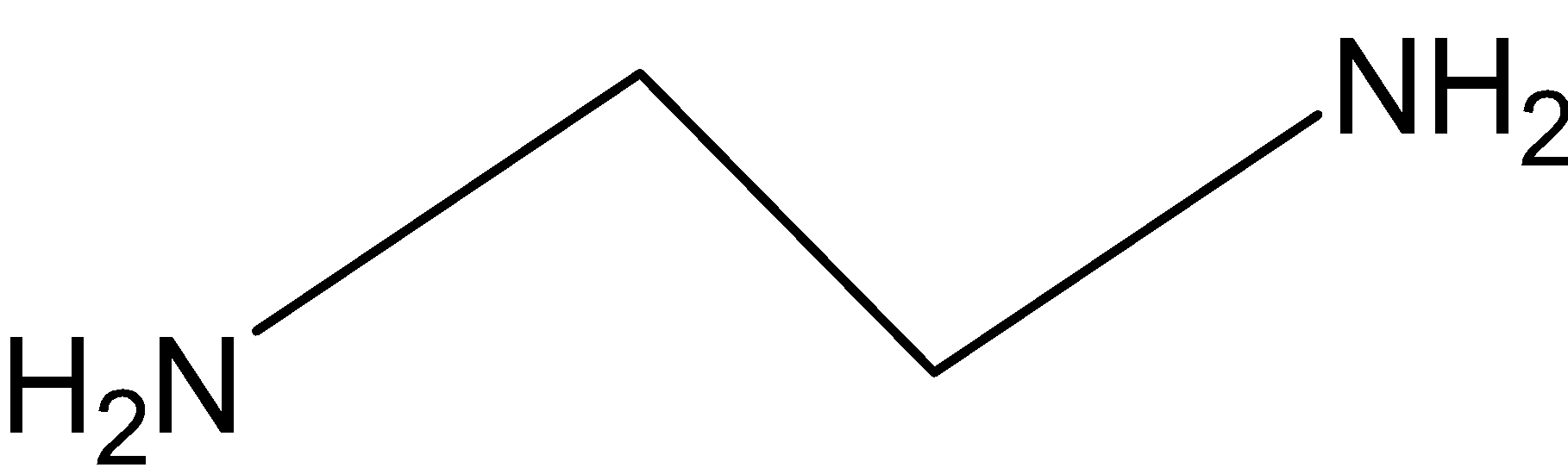
Figure 2: Ethylene diamine
Hence, the coordination number is \[{\text{2}}\].
For \[{\text{z}}\] value
The ligand superoxide (${{\text{O}}_{\text{2}}}^{\text{ - }}$) is a mono-dendate ligand. Hence, the coordination number is \[{\text{1}}\].
Since, the coordination number central metal atom is \[6\]. All these \[{\text{x, y and z}}\] values are equal to the \[6\].
${\text{2x + 2y + z = 6}}$………………………(1)
From Charge-balance equation:
${{\text{[Cr(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{x}}}{{\text{(en)}}_{\text{y}}}{{\text{(}}{{\text{O}}_{\text{2}}}{\text{)}}_{\text{z}}}{\text{]}}^{\text{ - }}}$
The ${\text{Cr}}$oxidation state ${\text{ + 3}}$, Oxalate ion (${{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$) oxidation state is ${\text{ - 2}}$ , ethylenediamine oxidation number is zero and superoxide ion oxidation number is ${\text{ - 1}}$.
$3 \times 1 + ( - 2) \times x + 0 \times y + ( - 1) \times z = - 1$
${\text{ - 2x - z = - 4}}$
${\text{2x + z = 4}}$………………………………..(2)
Substitute this value in equation (1)
${\text{4 + 2y = 6}}$
${\text{y = 1}}$
Now, since, \[{\text{x,y and z }}\]represent the number of ligands. Hence, it has an integral value and it cannot be zero.
Therefore, \[{\text{x = 1}}\].
\[{\text{x = 1}}\] and ${\text{y = 1}}$
Substitute these values in equation (1)
\[
{\text{2(1) + 2(1) + z = 6}} \\
{\text{4 + z = 6}} \\
{\text{z = 2}} \\
\]
Therefore, \[{\text{x = 1}}\],${\text{y = 1}}$ and \[{\text{z = 2}}\].
Hence, the correct option is \[{\text{2}}\].
Additional information:
-A coordination compound is basically a neutral species consisting of an uncoordinated ion and coordinated complexes to maintain the overall charge balance of a complex.
-In these compounds \[{\text{[ ]}}\] are mainly used to indicate the atomic composition of those are atoms and the ligands.
-Species which are present in the outside of \[{\text{[ ]}}\] are not coordinated to the central metal atom but these maintain overall charge of a complex.
Note:
Denticity of the ligand is the number of pairs of electrons shared with the metal atom. The poly-dendate ligands form the ring structure with the metal ion and hence contribute to extra stability. This is known as chelate effect.
Complete step by step answer:
The given coordination compounds are as follows.
${{\text{[Cr(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{x}}}{{\text{(en)}}_{\text{y}}}{{\text{(}}{{\text{O}}_{\text{2}}}{\text{)}}_{\text{z}}}{\text{]}}^{\text{ - }}}$
In this compound we have to find the \[{\text{x,y and z}}\] values.
The central metal atom – Chromium.
Chromium atomic number – \[{\text{24}}\]
Electronic configuration -\[{\text{[Ar]3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{2}}}\]. And it is ${\text{3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{2}}}$system. Hence, it has a total six valence electrons available for the formation of coordinate bonds.
For \[{\text{x}}\] value:
The ligand oxalate (${{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$) is a bi-dendate ligand means which donate two pairs of electrons to a central metal atom.

Figure 1: Oxalate ion
Hence, the coordination number is \[{\text{2}}\].
For \[{\text{y}}\] value:
The ligand ethylenediamine (en) is also bi-dendate ligand and it is a neutral ligand.

Figure 2: Ethylene diamine
Hence, the coordination number is \[{\text{2}}\].
For \[{\text{z}}\] value
The ligand superoxide (${{\text{O}}_{\text{2}}}^{\text{ - }}$) is a mono-dendate ligand. Hence, the coordination number is \[{\text{1}}\].
Since, the coordination number central metal atom is \[6\]. All these \[{\text{x, y and z}}\] values are equal to the \[6\].
${\text{2x + 2y + z = 6}}$………………………(1)
From Charge-balance equation:
${{\text{[Cr(}}{{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}{{\text{)}}_{\text{x}}}{{\text{(en)}}_{\text{y}}}{{\text{(}}{{\text{O}}_{\text{2}}}{\text{)}}_{\text{z}}}{\text{]}}^{\text{ - }}}$
The ${\text{Cr}}$oxidation state ${\text{ + 3}}$, Oxalate ion (${{\text{C}}_{\text{2}}}{{\text{O}}_{\text{4}}}^{{\text{2 - }}}$) oxidation state is ${\text{ - 2}}$ , ethylenediamine oxidation number is zero and superoxide ion oxidation number is ${\text{ - 1}}$.
$3 \times 1 + ( - 2) \times x + 0 \times y + ( - 1) \times z = - 1$
${\text{ - 2x - z = - 4}}$
${\text{2x + z = 4}}$………………………………..(2)
Substitute this value in equation (1)
${\text{4 + 2y = 6}}$
${\text{y = 1}}$
Now, since, \[{\text{x,y and z }}\]represent the number of ligands. Hence, it has an integral value and it cannot be zero.
Therefore, \[{\text{x = 1}}\].
\[{\text{x = 1}}\] and ${\text{y = 1}}$
Substitute these values in equation (1)
\[
{\text{2(1) + 2(1) + z = 6}} \\
{\text{4 + z = 6}} \\
{\text{z = 2}} \\
\]
Therefore, \[{\text{x = 1}}\],${\text{y = 1}}$ and \[{\text{z = 2}}\].
Hence, the correct option is \[{\text{2}}\].
Additional information:
-A coordination compound is basically a neutral species consisting of an uncoordinated ion and coordinated complexes to maintain the overall charge balance of a complex.
-In these compounds \[{\text{[ ]}}\] are mainly used to indicate the atomic composition of those are atoms and the ligands.
-Species which are present in the outside of \[{\text{[ ]}}\] are not coordinated to the central metal atom but these maintain overall charge of a complex.
Note:
Denticity of the ligand is the number of pairs of electrons shared with the metal atom. The poly-dendate ligands form the ring structure with the metal ion and hence contribute to extra stability. This is known as chelate effect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




