
How can you convert Newman projection for the staggered and eclipsed conformation of $2 - $Methylpentane for rotation about $$${C_2} - {C_3}$ bond to bond line notation$?$
Answer
539.7k+ views
Hint :First try to write down the formula of the given compound. Then draw the Newman projection of the molecule both in eclipsed and staggered conformation, eclipsed conformation is the one where two bulky groups are present in close proximity to each other i.e. the torsion angle between them is ${0^ \circ }$while in the staggered conformation the torsion angle between the two bulky groups is ${180^ \circ }$. Identify which group is above and below the plane and draw the bond-line notation.
Complete Step By Step Answer:
The given molecule is $2 - $Methylpentane which can be represented as:
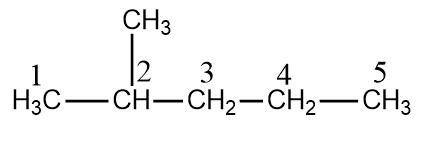
The numbering is done according to IUPAC, which states the $C$ atom having more substituent should be given more priority.
Now we need to draw the Newman projection for the compound in eclipsed and staggered form about the ${C_2} - {C_3}$ bond and convert to bond line notation.
Eclipsed conformation is the one where the two bulky groups are present in close proximity i.e. the torsion angle between them is ${0^ \circ }$while in the staggered conformation the torsion angle between the bulky groups is ${180^ \circ }$ and hence more stable.
In the given compound, ${C_2}$ atom has two methyl group and one hydrogen atom attached to it where methyl group is more bulky while in the ${C_3}$ atom there are two hydrogen atoms and one ethyl group attached where the ethyl group is more bulky.
So the Newman projection in eclipsed conformation will be:
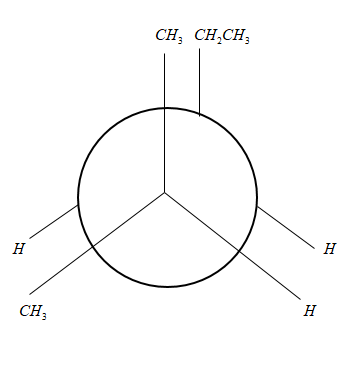
where ${C_2}$ is the front carbon and
${C_3}$ is the back carbon.
If we consider a line passing through the middle horizontally then the groups above the horizontal line are above the plane while those below are below the plane. Now if we tilt the bond line notation to the left then anything to the right is wedge and anything to the left is dashed. Hence the bond line notation for eclipsed conformation is:
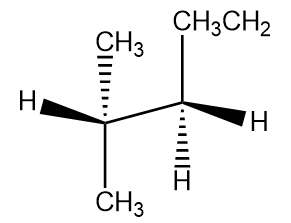
where ${C_2}$ is the front carbon and ${C_3}$ is the back carbon.
The Newman projection in staggered conformation will be:
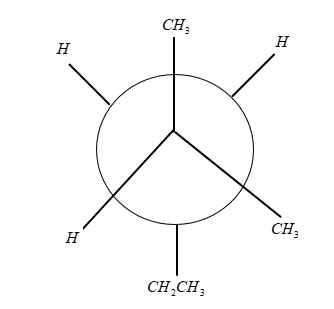
where ${C_2}$ is the front carbon and ${C_3}$ is the back carbon.
Following the same rule the bond line notation for staggered conformation is:
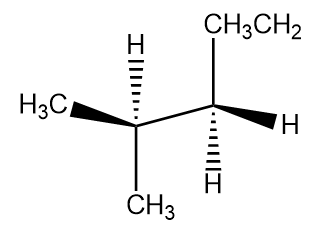
where ${C_2}$ is the front carbon and ${C_3}$ is the back carbon.
Note :
Make sure to number the compound following IUPAC as it may lead to error if not followed. Also remember there are various possible ways via which you can represent a molecule so do not confuse among them. Due to the conversion of one projection to another very carefully considering which group is above and below the plane.
Complete Step By Step Answer:
The given molecule is $2 - $Methylpentane which can be represented as:

The numbering is done according to IUPAC, which states the $C$ atom having more substituent should be given more priority.
Now we need to draw the Newman projection for the compound in eclipsed and staggered form about the ${C_2} - {C_3}$ bond and convert to bond line notation.
Eclipsed conformation is the one where the two bulky groups are present in close proximity i.e. the torsion angle between them is ${0^ \circ }$while in the staggered conformation the torsion angle between the bulky groups is ${180^ \circ }$ and hence more stable.
In the given compound, ${C_2}$ atom has two methyl group and one hydrogen atom attached to it where methyl group is more bulky while in the ${C_3}$ atom there are two hydrogen atoms and one ethyl group attached where the ethyl group is more bulky.
So the Newman projection in eclipsed conformation will be:

where ${C_2}$ is the front carbon and
${C_3}$ is the back carbon.
If we consider a line passing through the middle horizontally then the groups above the horizontal line are above the plane while those below are below the plane. Now if we tilt the bond line notation to the left then anything to the right is wedge and anything to the left is dashed. Hence the bond line notation for eclipsed conformation is:

where ${C_2}$ is the front carbon and ${C_3}$ is the back carbon.
The Newman projection in staggered conformation will be:

where ${C_2}$ is the front carbon and ${C_3}$ is the back carbon.
Following the same rule the bond line notation for staggered conformation is:

where ${C_2}$ is the front carbon and ${C_3}$ is the back carbon.
Note :
Make sure to number the compound following IUPAC as it may lead to error if not followed. Also remember there are various possible ways via which you can represent a molecule so do not confuse among them. Due to the conversion of one projection to another very carefully considering which group is above and below the plane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




