
How will you convert
(i) Calcium acetate and acetaldehyde
(ii) Acetone to acetone cyanohydrin
(iii) Sodium acetate to methane
(iv) Benzoic acid to $m - $ bromobenzoic acid
Answer
526.8k+ views
Hint: A chemical reaction is defined as a process in which the bond of the reactant molecules breaks and results in the formation of a new bond that gives a product molecule. It is a reaction in which two or more molecules interact and form new substances.
Complete step by step solution:
(i) Calcium acetate is a chemical compound that consists of calcium salt or acetic acid. Preparation of calcium acetate can be done by soaking calcium carbonate. It is used as a stabiliser, food additive. When dry distillation of calcium acetate takes place, it results in the formation of acetaldehyde and calcium carbonate.
$C{H_3}COO - Ca - C{H_3}COO + HCOO - Ca - OOCH \to 2C{H_3}CHO + 2CaC{O_3}$
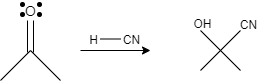
(ii) Conversion of acetone to acetone cyanohydrin is a nucleophilic addition process. In this reaction, acetone is reacted with hydrogen cyanohydrin, in which the oxygen atom of the acetone gets attached with hydrogen and the carbon atom of acetone gets attached with cyanide. This results in the formation of acetone cyanohydrin.
(iii) Sodium acetate is a hygroscopic powder which is very soluble in water. When sodium acetate is heated with soda lime, it results in the formation of methane and sodium carbonate. Soda lime is prepared when sodium hydroxide is reacted with calcium oxide.
$C{H_3} - \mathop C\limits^{\mathop \parallel \limits^O } - {O^ - }N{a^ + }\xrightarrow[{CaO}]{{NaOH}}C{H_4} + C{O_2}$
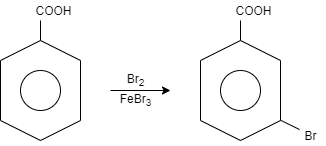
(iv) When benzoic acid is reacted with bromine in presence of a Lewis acid catalyst that is iron bromide, it results in the formation of $m - $bromobenzoic acid. This catalyst is used for the halogenations of aromatic compounds.
Note:
In this question we concluded that a chemical reaction takes place when a substance is converted into another substance. We should also note that nucleophilic addition reaction takes place when a chemical compound that contains electrophilic double or triple bond reacts with nucleophile in such a way that double or triple bond is broken.
Complete step by step solution:
(i) Calcium acetate is a chemical compound that consists of calcium salt or acetic acid. Preparation of calcium acetate can be done by soaking calcium carbonate. It is used as a stabiliser, food additive. When dry distillation of calcium acetate takes place, it results in the formation of acetaldehyde and calcium carbonate.
$C{H_3}COO - Ca - C{H_3}COO + HCOO - Ca - OOCH \to 2C{H_3}CHO + 2CaC{O_3}$
(ii) Conversion of acetone to acetone cyanohydrin is a nucleophilic addition process. In this reaction, acetone is reacted with hydrogen cyanohydrin, in which the oxygen atom of the acetone gets attached with hydrogen and the carbon atom of acetone gets attached with cyanide. This results in the formation of acetone cyanohydrin.

(iii) Sodium acetate is a hygroscopic powder which is very soluble in water. When sodium acetate is heated with soda lime, it results in the formation of methane and sodium carbonate. Soda lime is prepared when sodium hydroxide is reacted with calcium oxide.
$C{H_3} - \mathop C\limits^{\mathop \parallel \limits^O } - {O^ - }N{a^ + }\xrightarrow[{CaO}]{{NaOH}}C{H_4} + C{O_2}$
(iv) When benzoic acid is reacted with bromine in presence of a Lewis acid catalyst that is iron bromide, it results in the formation of $m - $bromobenzoic acid. This catalyst is used for the halogenations of aromatic compounds.

Note:
In this question we concluded that a chemical reaction takes place when a substance is converted into another substance. We should also note that nucleophilic addition reaction takes place when a chemical compound that contains electrophilic double or triple bond reacts with nucleophile in such a way that double or triple bond is broken.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




