
How to convert benzoic acid to benzene?
Answer
524.4k+ views
Hint: The conversion of benzoic acid to benzene is an example of decarboxylation reaction. The structure of benzoic acid and benzene are as follows.
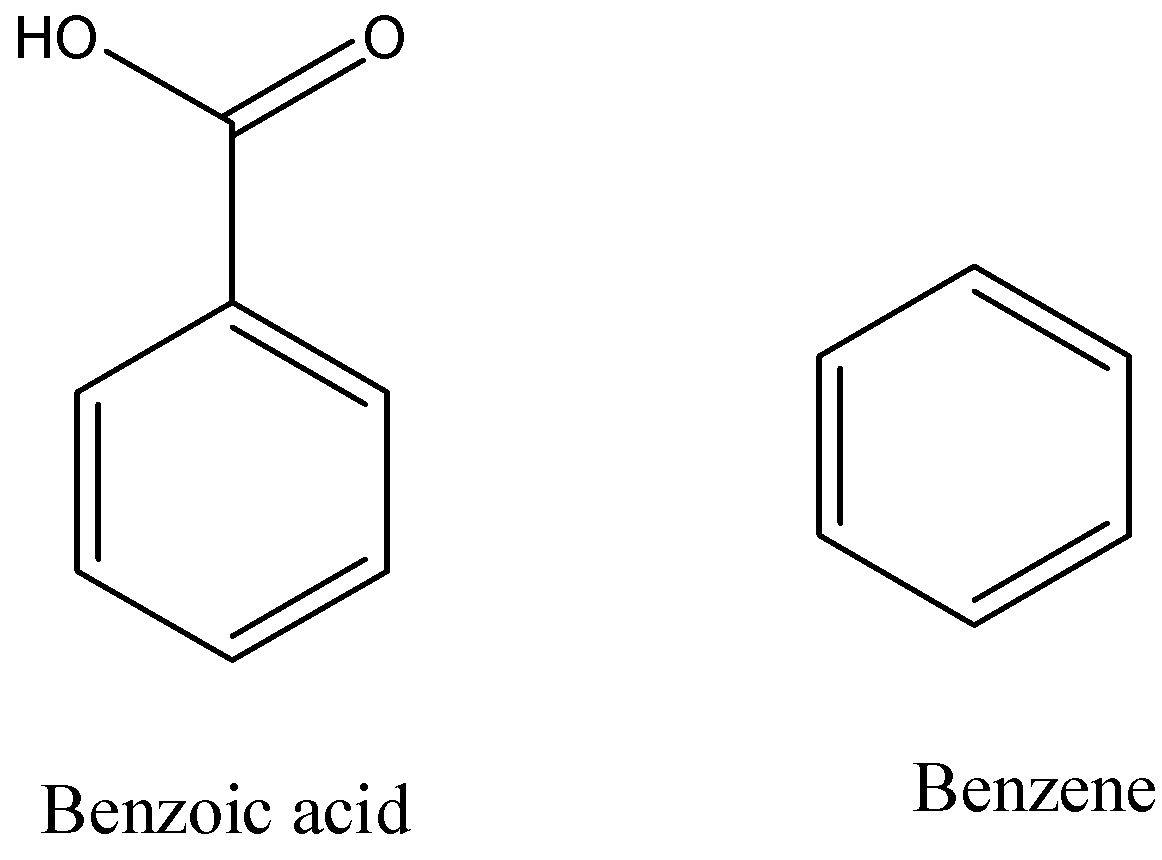
Complete answer:
- In the question it asked to convert the benzoic acid to benzene.
- The conversion of benzoic acid to benzene is a two-step process.
- In the first step benzoic acid is going to convert into sodium benzoate and in the second step the sodium benzoate is going to be converted into benzene on reaction with water.
Step-1:
- In the first step the conversion of benzoic acid to sodium benzoate is going to occur by the usage of the sodium hydroxide in the presence of calcium oxide.
- The combination of sodium hydroxide with calcium oxide is known as soda lime.
- The chemical reaction of converting benzoic acid to sodium benzoate in the presence of soda lime is as follows.
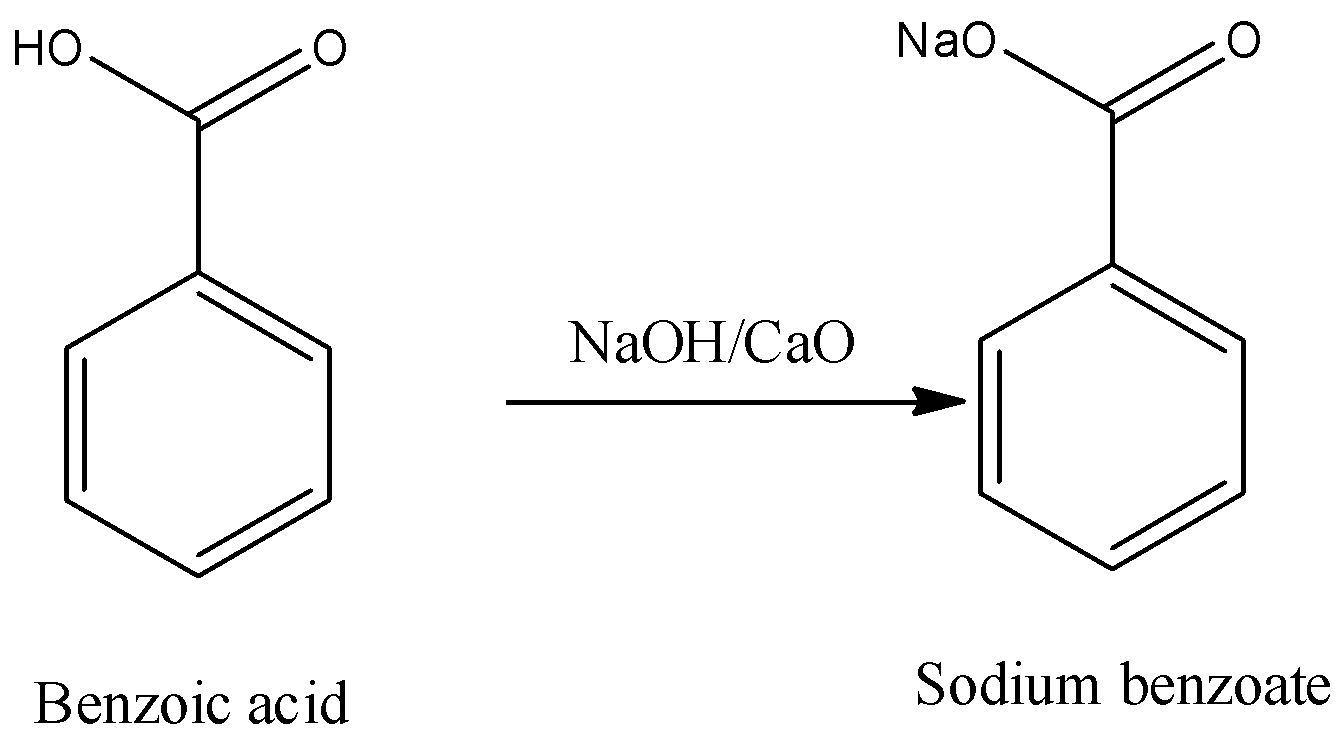
Step-2:
- In this step the sodium benzoate will undergo hydrolysis with water and forms benzene as a product.
- The chemical reaction of sodium benzoate into benzene is as follows.
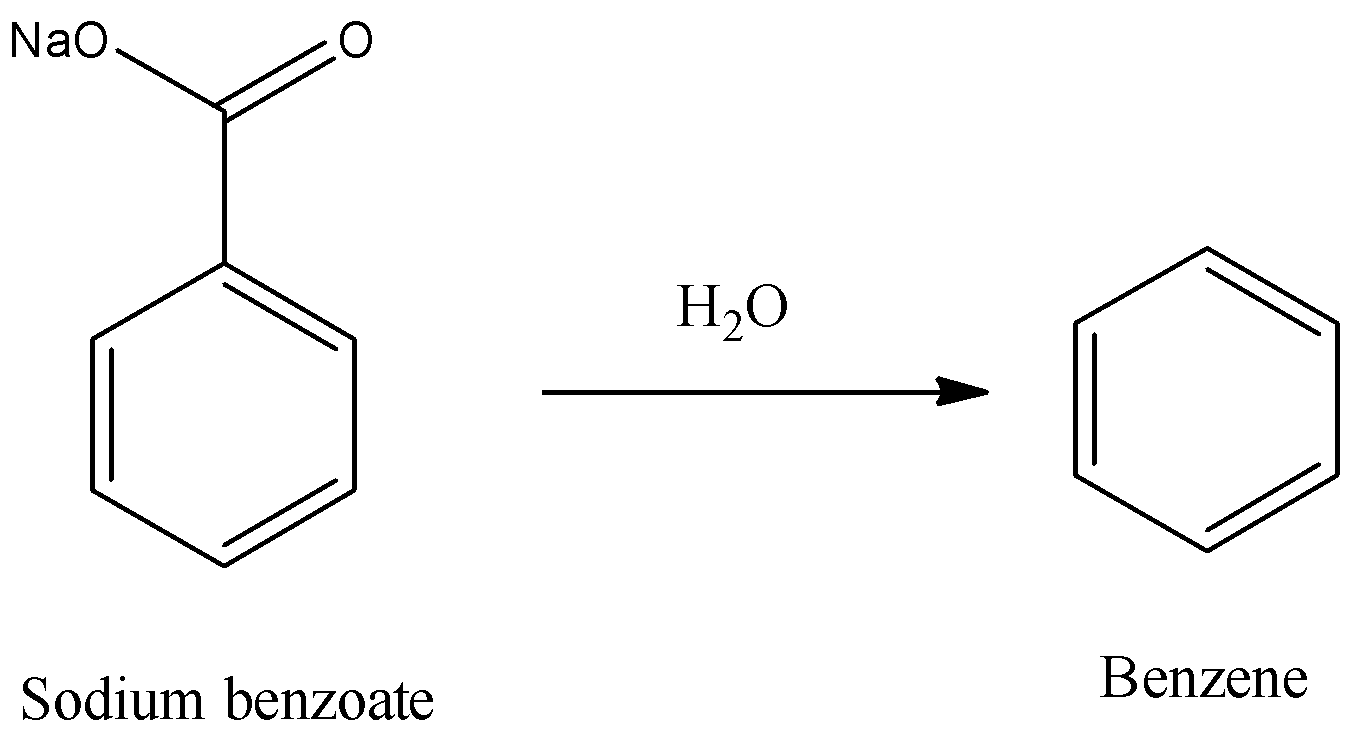
Note:
All the organic compounds that contain carboxylic acid functional groups easily undergo decarboxylation and form the respective hydrocarbon as the product. We can prepare benzene from phenol also. In the conversion of phenol to benzene there is a role of zinc dust in the presence of heat. That means benzene can be prepared in two ways.

Complete answer:
- In the question it asked to convert the benzoic acid to benzene.
- The conversion of benzoic acid to benzene is a two-step process.
- In the first step benzoic acid is going to convert into sodium benzoate and in the second step the sodium benzoate is going to be converted into benzene on reaction with water.
Step-1:
- In the first step the conversion of benzoic acid to sodium benzoate is going to occur by the usage of the sodium hydroxide in the presence of calcium oxide.
- The combination of sodium hydroxide with calcium oxide is known as soda lime.
- The chemical reaction of converting benzoic acid to sodium benzoate in the presence of soda lime is as follows.

Step-2:
- In this step the sodium benzoate will undergo hydrolysis with water and forms benzene as a product.
- The chemical reaction of sodium benzoate into benzene is as follows.

Note:
All the organic compounds that contain carboxylic acid functional groups easily undergo decarboxylation and form the respective hydrocarbon as the product. We can prepare benzene from phenol also. In the conversion of phenol to benzene there is a role of zinc dust in the presence of heat. That means benzene can be prepared in two ways.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




