
How will you convert aniline into:
(a)- Benzyl alcohol
(b)- 4-Bromoaniline
(c)- 1, 3, 5-Tribromobenzene
(d)- 2, 4, 6- Tribromofluorobenzene
(e)- 4-Nitroaniline
(f)- Sulphanilic acid
Answer
512.7k+ views
Hint: Aniline is an organic compound in which the $-N{{H}_{2}}$ group is attached to the benzene ring. In all the conversions above, multi-step reactions are required to get the desired product. Aniline can be treated with nitrous acid to give diazonium salt.
Complete answer:
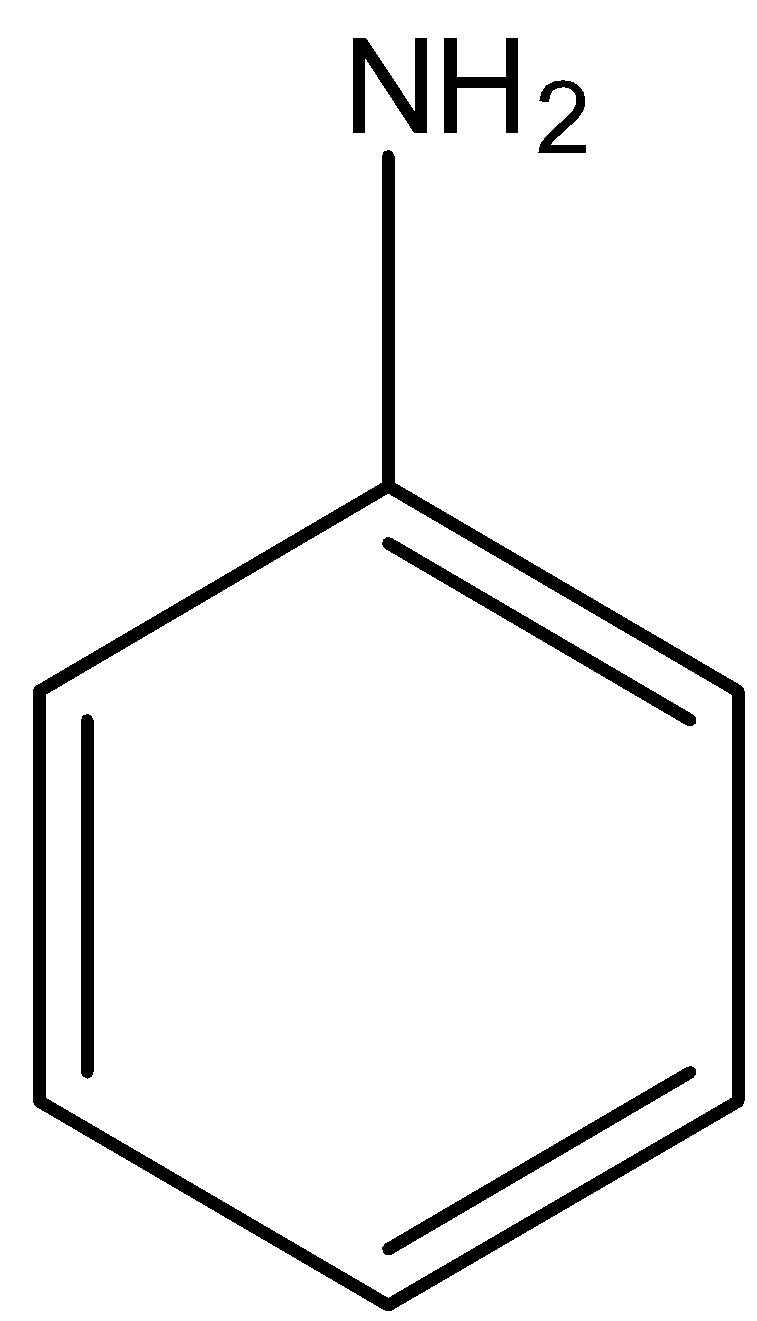
When the $-N{{H}_{2}}$ group is attached to the benzene ring then it is known as aniline. Its structure is given below:
(a)- Benzyl alcohol
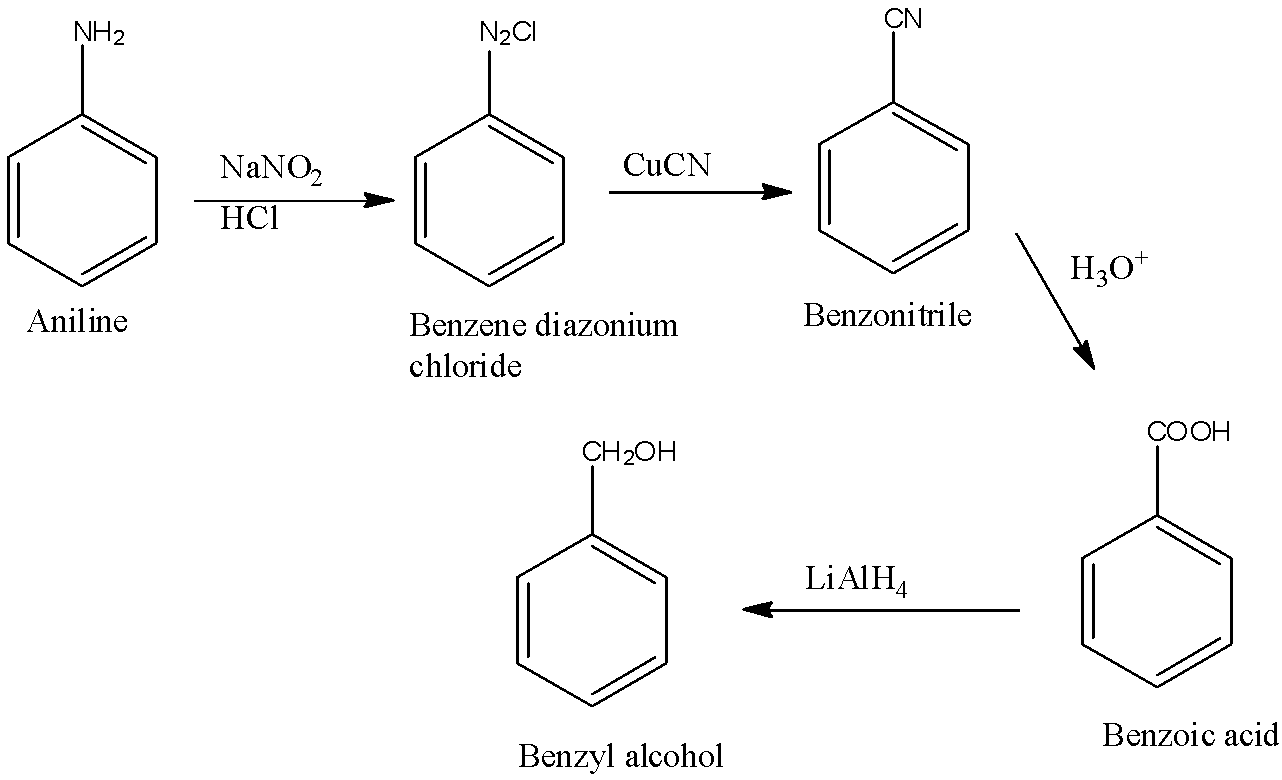
When $-C{{H}_{2}}OH$ is attached to the benzene then it is known as benzyl alcohol. First, aniline will be converted into benzene diazonium chloride. Benzene diazonium chloride will be converted into benzonitrile. This benzonitrile is converted into benzoic acid and the benzoic acid is converted into benzyl alcohol. The reactions are given below:
(b)- 4-Bromonitrile
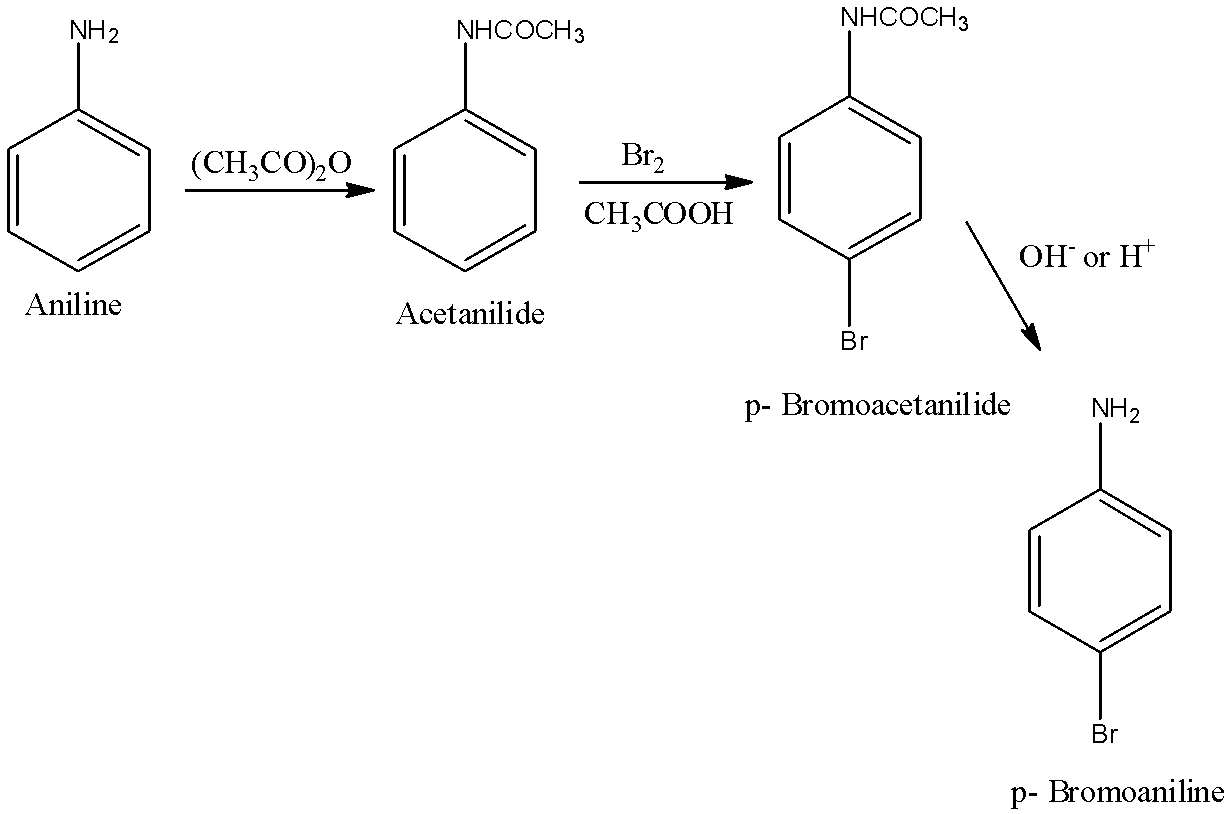
When the first position in the benzene has the $-N{{H}_{2}}$ group and the fourth position has –Br, then it is called 4-Bromonitrile. First, aniline will be converted into Acetanilide. Acetanilide will be converted into p-Bromoacetanilide and this will be further into p-Bromoaniline or 4-Bromoaniline. The reactions are given below:
(c)- 1, 3, 5-Tribromobenzene
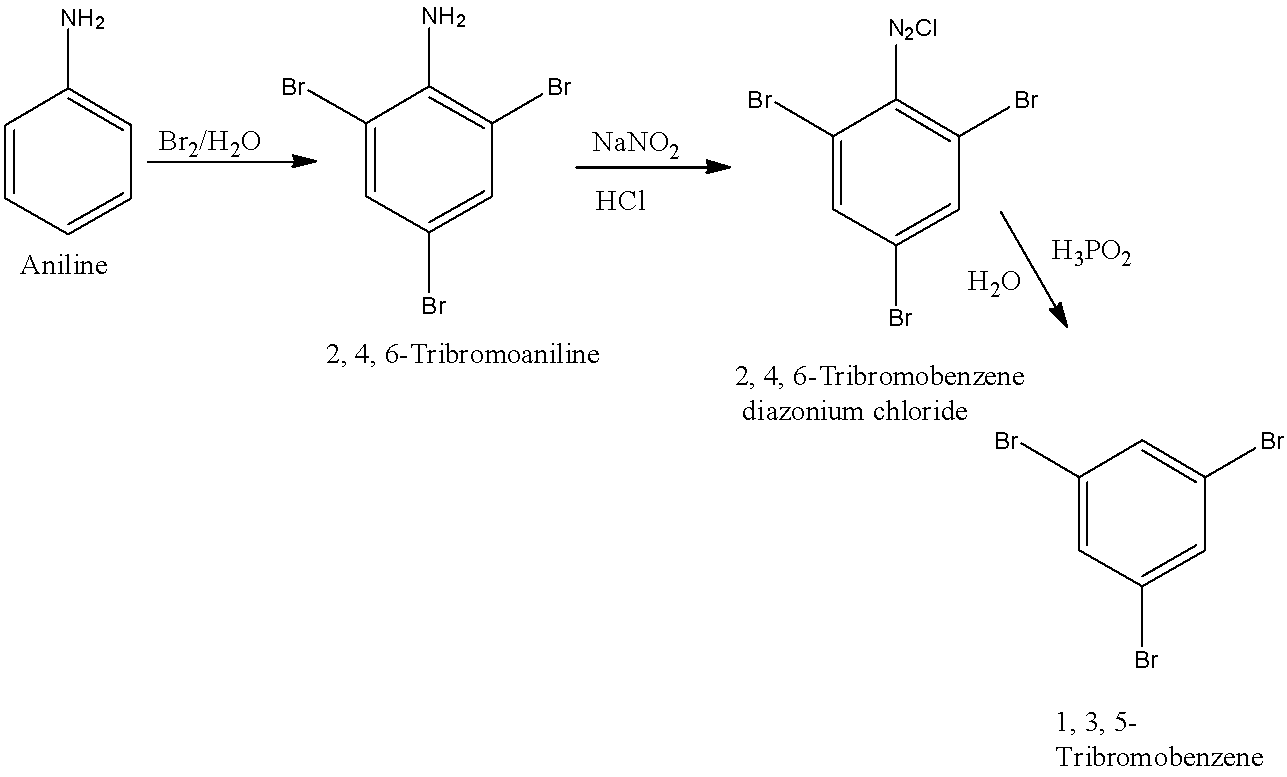
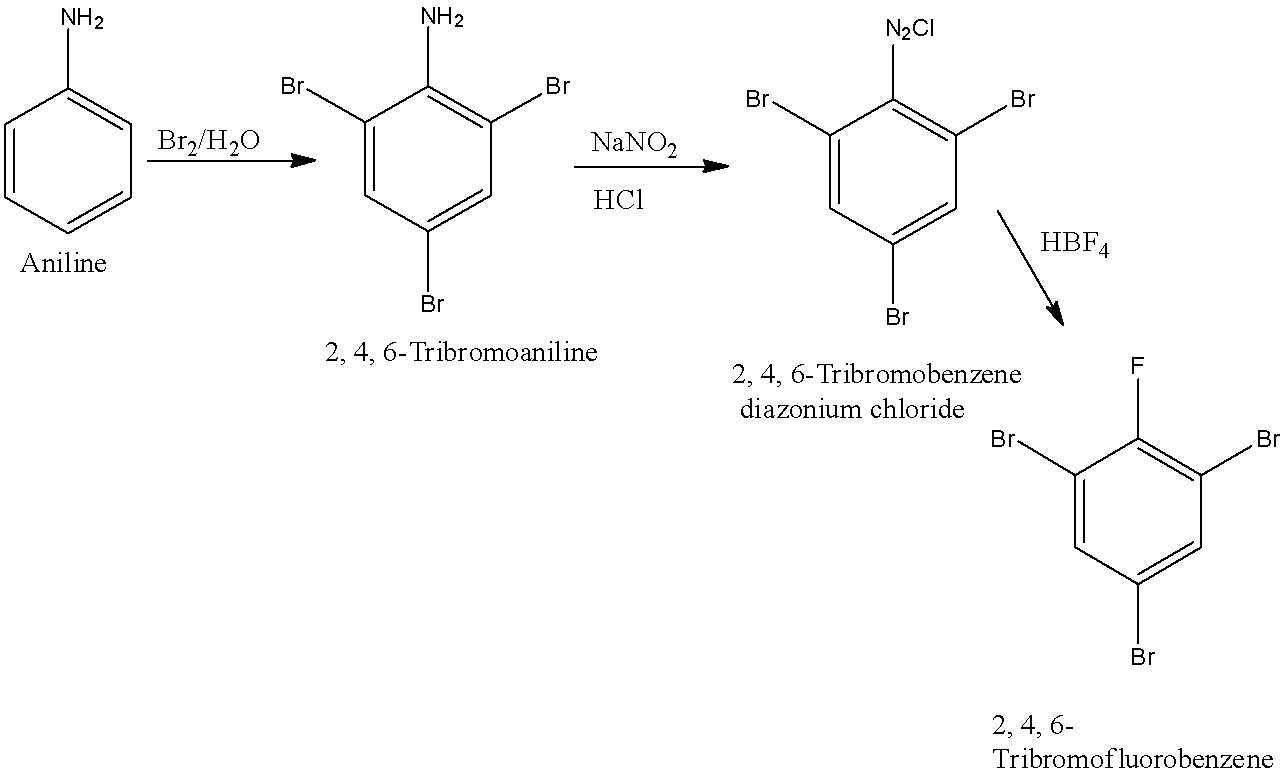
When the 1st, 3rd, and 5th position in the benzene is occupied by bromine then it is known as 1, 3, 5-Tribromoaniline. First, aniline will be converted into 2, 4, 6-Tribromoaniline. 2, 4, 6-Tribromoaniline will be converted into 2, 4, 6-Tribromobenzene diazonium chloride and it will be converted into 1, 3, 5-Tribromobenzene. The reactions are given below:
(d)- 2, 4, 6-Tribromofluorobenzene
When 1st position of the benzene ring is occupied by fluorine while 2nd, 4th, and 6th positions are occupied by bromine then it is known as 2, 4, 6-Tribromofluorobenzene. First, aniline will be converted into 2, 4, 6-Tribromoaniline. 2, 4, 6-Tribromoaniline will be converted into 2, 4, 6-Tribromobenzene diazonium chloride and it will be converted into 2, 4, 6-Tribromofluorobenzene. The reactions are given below:
(e)- 4-Nitroaniline
When the 4th position of the benzene contains $-N{{O}_{2}}$ and at the first position there is $-N{{H}_{2}}$ group. First, aniline will be converted into Acetanilide. Acetanilide will be converted into p-Nitroacetanilide and this will be further into p-Nitroaniline or 4-Nitroaniline. The reactions are given below:

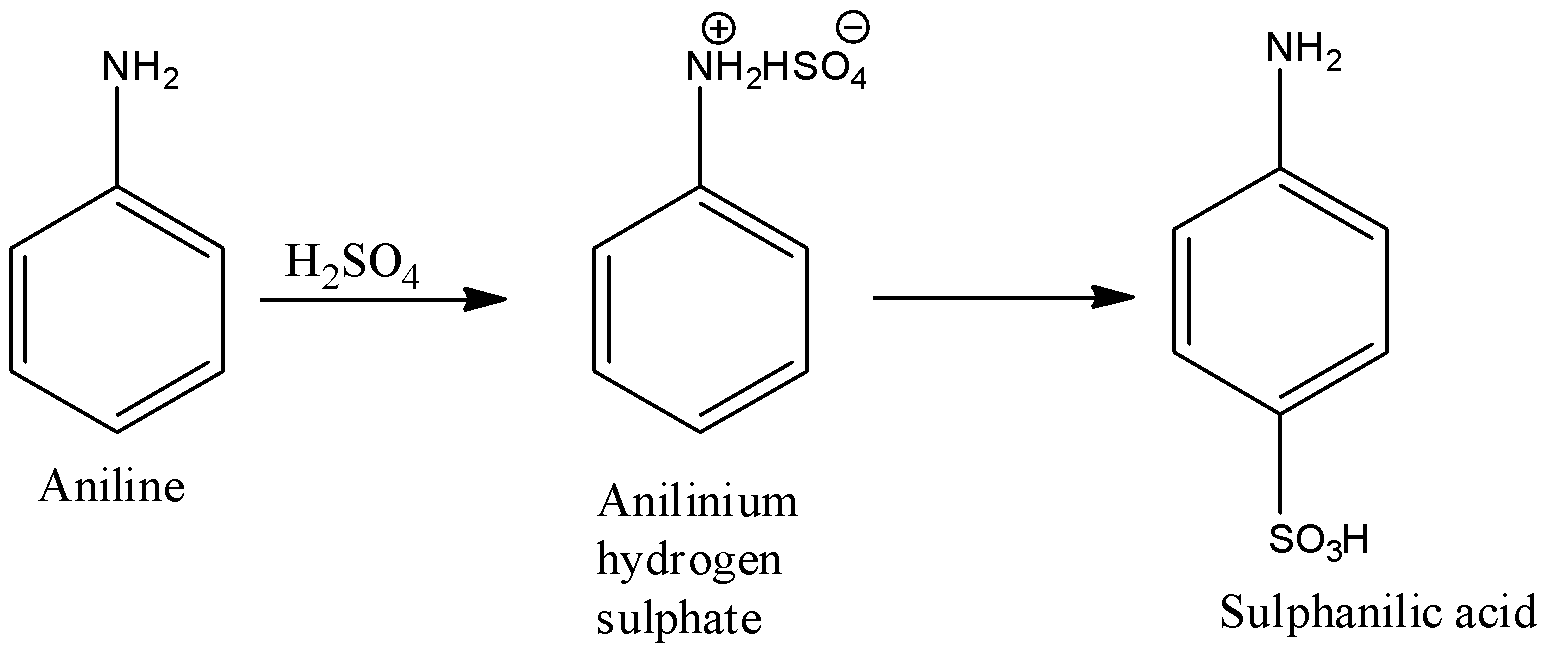
(f)- Sulphanilic acid
When the 4th position of the benzene ring is occupied by $-S{{O}_{3}}H$ and the first position is occupied by $-N{{H}_{2}}$ group. First, aniline is converted into anilinium hydrogen sulfate, and then it is converted into sulphanilic acid. The reactions are given below:
Note:
The sulphanilic acid doesn’t remain as such and it is in equilibrium with its zwitterion form, i.e., the hydrogen ion from the $-S{{O}_{3}}H$ moves towards the $-N{{H}_{2}}$ group. It is given below:

Complete answer:
When the $-N{{H}_{2}}$ group is attached to the benzene ring then it is known as aniline. Its structure is given below:

(a)- Benzyl alcohol
When $-C{{H}_{2}}OH$ is attached to the benzene then it is known as benzyl alcohol. First, aniline will be converted into benzene diazonium chloride. Benzene diazonium chloride will be converted into benzonitrile. This benzonitrile is converted into benzoic acid and the benzoic acid is converted into benzyl alcohol. The reactions are given below:

(b)- 4-Bromonitrile
When the first position in the benzene has the $-N{{H}_{2}}$ group and the fourth position has –Br, then it is called 4-Bromonitrile. First, aniline will be converted into Acetanilide. Acetanilide will be converted into p-Bromoacetanilide and this will be further into p-Bromoaniline or 4-Bromoaniline. The reactions are given below:

(c)- 1, 3, 5-Tribromobenzene
When the 1st, 3rd, and 5th position in the benzene is occupied by bromine then it is known as 1, 3, 5-Tribromoaniline. First, aniline will be converted into 2, 4, 6-Tribromoaniline. 2, 4, 6-Tribromoaniline will be converted into 2, 4, 6-Tribromobenzene diazonium chloride and it will be converted into 1, 3, 5-Tribromobenzene. The reactions are given below:

(d)- 2, 4, 6-Tribromofluorobenzene
When 1st position of the benzene ring is occupied by fluorine while 2nd, 4th, and 6th positions are occupied by bromine then it is known as 2, 4, 6-Tribromofluorobenzene. First, aniline will be converted into 2, 4, 6-Tribromoaniline. 2, 4, 6-Tribromoaniline will be converted into 2, 4, 6-Tribromobenzene diazonium chloride and it will be converted into 2, 4, 6-Tribromofluorobenzene. The reactions are given below:

(e)- 4-Nitroaniline
When the 4th position of the benzene contains $-N{{O}_{2}}$ and at the first position there is $-N{{H}_{2}}$ group. First, aniline will be converted into Acetanilide. Acetanilide will be converted into p-Nitroacetanilide and this will be further into p-Nitroaniline or 4-Nitroaniline. The reactions are given below:

(f)- Sulphanilic acid
When the 4th position of the benzene ring is occupied by $-S{{O}_{3}}H$ and the first position is occupied by $-N{{H}_{2}}$ group. First, aniline is converted into anilinium hydrogen sulfate, and then it is converted into sulphanilic acid. The reactions are given below:

Note:
The sulphanilic acid doesn’t remain as such and it is in equilibrium with its zwitterion form, i.e., the hydrogen ion from the $-S{{O}_{3}}H$ moves towards the $-N{{H}_{2}}$ group. It is given below:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




