
How can I convert a Newman projection for ethane molecule to bond line notation?
Answer
558.9k+ views
Hint: To solve this we must know that the molecular formula for ethane is ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}$. The Newman projection visualizes the conformation of a chemical bond from front to back. In Newman projection, the front atom is represented by a dot and the back atom is represented by a circle.
Complete answer:
We know that the molecular formula for ethane is ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}$.
The Newman projection visualizes the conformation of a chemical bond from front to back. In Newman projection, the front carbon atom is represented by a dot and the back carbon atom is represented by a circle.
Thus, the Newman projection for ethane molecule is as follows:
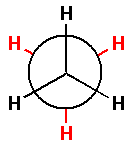
In the Newman projection for ethane molecules, the black hydrogen atoms are attached to the front carbon atom and the red hydrogen atoms are attached to the back carbon atom.
In bond line notation, the bond between two atoms is denoted by a line. In bond line notation, atoms other than carbon and hydrogen are shown.
The steps to convert Newman projection to bond line notation are as follows:
Find the longest chain of carbon atoms.
Locate the substituents.
Now, in the Newman projection for ethane molecules, the longest chain of carbon atoms has two carbon atoms. And each carbon atom has three hydrogen atoms as substituents.
Thus, the bond line notation for ethane molecule is as follows:
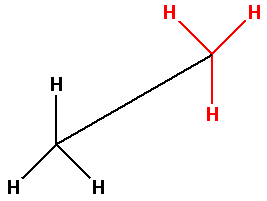
Note:Remember the steps to convert Newman projection to bond line notation are: 1. Find the longest chain of carbon atoms, 2. Locate the substituents. In bond line notation, the bond between two atoms is denoted by a line and only the atoms other than carbon and hydrogen are shown.
Complete answer:
We know that the molecular formula for ethane is ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}$.
The Newman projection visualizes the conformation of a chemical bond from front to back. In Newman projection, the front carbon atom is represented by a dot and the back carbon atom is represented by a circle.
Thus, the Newman projection for ethane molecule is as follows:

In the Newman projection for ethane molecules, the black hydrogen atoms are attached to the front carbon atom and the red hydrogen atoms are attached to the back carbon atom.
In bond line notation, the bond between two atoms is denoted by a line. In bond line notation, atoms other than carbon and hydrogen are shown.
The steps to convert Newman projection to bond line notation are as follows:
Find the longest chain of carbon atoms.
Locate the substituents.
Now, in the Newman projection for ethane molecules, the longest chain of carbon atoms has two carbon atoms. And each carbon atom has three hydrogen atoms as substituents.
Thus, the bond line notation for ethane molecule is as follows:

Note:Remember the steps to convert Newman projection to bond line notation are: 1. Find the longest chain of carbon atoms, 2. Locate the substituents. In bond line notation, the bond between two atoms is denoted by a line and only the atoms other than carbon and hydrogen are shown.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




