
How many constitutional isomers does${{C}_{3}}{{H}_{8}}O$ have?
Answer
493.2k+ views
Hint: Isomers are compounds having the same molecular formula but different structural formula. Isomers can be of various types like, geometrical, constitutional, stereoisomer etc. constitutional isomers contain different positions of the alkyl groups, and these are same as structural isomers.
Complete answer:
Isomers are defined as the compounds having the same chemical formula but having different structural representations. There are various types of isomers; among them are the constitutional isomers that have the same molecular formula but the position of the functional groups or the alkyl group changes.
We have been given a compound that has 3 carbon, 8 hydrogen and 1 oxygen, as${{C}_{3}}{{H}_{8}}O$, as the general formula of alcohols suggests that${{C}_{n}}{{H}_{2n+1}}OH$ a 3 carbon alcohol, or propanol will have${{C}_{3}}{{H}_{7}}OH$ that is${{C}_{3}}{{H}_{8}}O$. So, the compound can be known as an alcohol.
For alcohol, propanol can exist in 2 constitutional isomers that are:
1-propanol whose structure has OH functional group at 1st carbon as
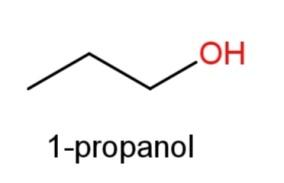 and
and
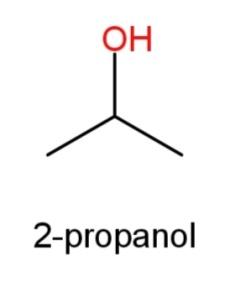
2-propanol whose structure has OH group at the second carbon atom as
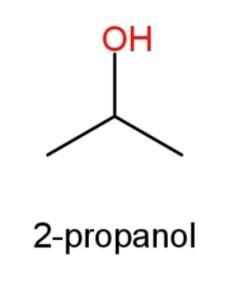
Now, a 3rd isomer is also possible when we do not consider OH functional group, and consider an ether as a functional group that has formula R – O – R, where R is the alkyl group, so with 3 carbon it can be:
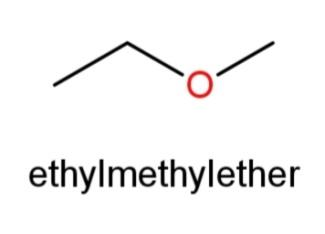
Ethyl methyl ether whose structure has O in the center and an ethyl and methyl group at the ends as,
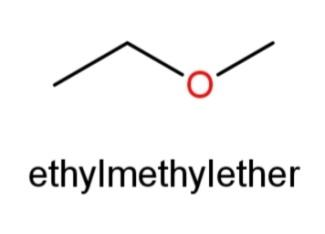
Hence, 3 constitutional isomers of${{C}_{3}}{{H}_{8}}O$are possible that are
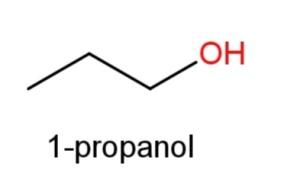 ,
,
 ’
’
 .
.
Note:
The constitutional isomers can be of various types, here we have made functional isomers in which the position of functional groups changes the structures. When the carbon skeleton is rearranged then it is called skeletal isomers, when the alkyl groups are made as straight chains or taken as branches then they are termed as positional isomers.
Complete answer:
Isomers are defined as the compounds having the same chemical formula but having different structural representations. There are various types of isomers; among them are the constitutional isomers that have the same molecular formula but the position of the functional groups or the alkyl group changes.
We have been given a compound that has 3 carbon, 8 hydrogen and 1 oxygen, as${{C}_{3}}{{H}_{8}}O$, as the general formula of alcohols suggests that${{C}_{n}}{{H}_{2n+1}}OH$ a 3 carbon alcohol, or propanol will have${{C}_{3}}{{H}_{7}}OH$ that is${{C}_{3}}{{H}_{8}}O$. So, the compound can be known as an alcohol.
For alcohol, propanol can exist in 2 constitutional isomers that are:
1-propanol whose structure has OH functional group at 1st carbon as

2-propanol whose structure has OH group at the second carbon atom as

Now, a 3rd isomer is also possible when we do not consider OH functional group, and consider an ether as a functional group that has formula R – O – R, where R is the alkyl group, so with 3 carbon it can be:
Ethyl methyl ether whose structure has O in the center and an ethyl and methyl group at the ends as,

Hence, 3 constitutional isomers of${{C}_{3}}{{H}_{8}}O$are possible that are



Note:
The constitutional isomers can be of various types, here we have made functional isomers in which the position of functional groups changes the structures. When the carbon skeleton is rearranged then it is called skeletal isomers, when the alkyl groups are made as straight chains or taken as branches then they are termed as positional isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




