
How many constitutional isomers are possible for the formula ${{C}_{4}}{{H}_{9}}Cl$?
Answer
558.9k+ views
Hint: Isomers with the same molecular formula but different connectivity between atoms are called constitutional isomers.
- The isomers formed will be position isomers.
Complete step by step answer:
- So in the question it is given that how many constitutional isomers will be possible for the given molecule. First we have to brush up the concept of isomerism so that we could get a better idea for drawing the isomeric structures.
- Isomers are those molecules which have the same molecular formula but will have the difference in arrangement of the atoms.
- If the isomers differ in connectivity of atoms and yield different structures, then that isomers are called as the structural isomers or constitutional isomers and if the arrangement of atoms differ in space, they are called as the stereoisomers.
- So now we know that the constitutional isomers are structural isomers and there are many types of conformational isomers. They are:
-Position isomers- the position of functional groups will be different with same parent carbon skeleton
-Chain isomers-same molecular formula but differ in the parent C chain
-Functional isomers-same molecular formulae and carbon chain but differ in the functional group present in them etc.
- Now let’s discuss the molecule given about, since in the formulae there are four C atoms, there is a chance that the main C chain may be butane.
- For that check the general formulae that satisfies the condition for alkane, i.e. alkane will have the general formulae ${{C}_{n}}{{H}_{2n+2}}$
Here the molecule satisfies the formulae, it should have a molecule formulae of ${{C}_{4}}{{H}_{10}}$in which one H is replaced by the Cl atoms.
Now let’s write the normal chain of butane and substitute the Cl atom in C-1. The structure of isomer obtained will be,

Now shift the position of the Cl atom to second carbon atom, so that a position isomer will be formed. The position of the substituent in the carbon chain is changed.

Now shift the position of the methyl group, insert the methyl group in the second carbon, which will yield a secondary structure of butane and the parent carbon will be propane. The structure formed is,
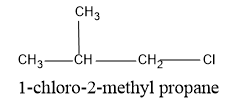
Now shift the Cl atom also to the second carbon in the propane chain so that the structure formed is a tertiary structure of the butane parent chain.
The structure formed will be as follows,
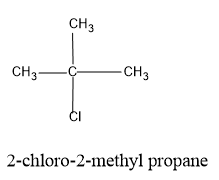
Note: While drawing the structure of isomers always verify that the valency of C and other atoms are satisfied.
- And while tracing these structures and naming the isomers the parent carbon, all the carbon chain should be numbered in a similar fashion, the numbering should not change from one structure to another i.e. if one structure is numbered from left to right then follow it in that fashion to avoid confusion while drawing the structures of isomers.
- The isomers formed will be position isomers.
Complete step by step answer:
- So in the question it is given that how many constitutional isomers will be possible for the given molecule. First we have to brush up the concept of isomerism so that we could get a better idea for drawing the isomeric structures.
- Isomers are those molecules which have the same molecular formula but will have the difference in arrangement of the atoms.
- If the isomers differ in connectivity of atoms and yield different structures, then that isomers are called as the structural isomers or constitutional isomers and if the arrangement of atoms differ in space, they are called as the stereoisomers.
- So now we know that the constitutional isomers are structural isomers and there are many types of conformational isomers. They are:
-Position isomers- the position of functional groups will be different with same parent carbon skeleton
-Chain isomers-same molecular formula but differ in the parent C chain
-Functional isomers-same molecular formulae and carbon chain but differ in the functional group present in them etc.
- Now let’s discuss the molecule given about, since in the formulae there are four C atoms, there is a chance that the main C chain may be butane.
- For that check the general formulae that satisfies the condition for alkane, i.e. alkane will have the general formulae ${{C}_{n}}{{H}_{2n+2}}$
Here the molecule satisfies the formulae, it should have a molecule formulae of ${{C}_{4}}{{H}_{10}}$in which one H is replaced by the Cl atoms.
Now let’s write the normal chain of butane and substitute the Cl atom in C-1. The structure of isomer obtained will be,

Now shift the position of the Cl atom to second carbon atom, so that a position isomer will be formed. The position of the substituent in the carbon chain is changed.

Now shift the position of the methyl group, insert the methyl group in the second carbon, which will yield a secondary structure of butane and the parent carbon will be propane. The structure formed is,

Now shift the Cl atom also to the second carbon in the propane chain so that the structure formed is a tertiary structure of the butane parent chain.
The structure formed will be as follows,

Note: While drawing the structure of isomers always verify that the valency of C and other atoms are satisfied.
- And while tracing these structures and naming the isomers the parent carbon, all the carbon chain should be numbered in a similar fashion, the numbering should not change from one structure to another i.e. if one structure is numbered from left to right then follow it in that fashion to avoid confusion while drawing the structures of isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




