
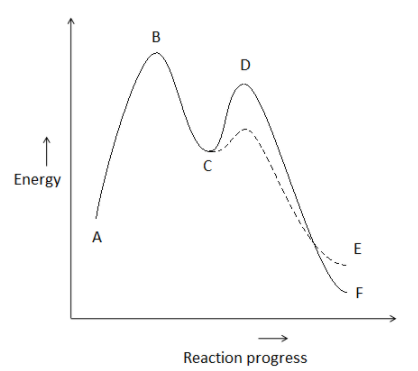
Consider the potential energy diagram given below. In accordance with Hammond’s postulate, exothermic reactions tend to have _________.
(A) Early transition states that are reactant like
(B) Late transition states that are reactant like
(C) Early transition states that are product like
(D) Late transition states that are product like

Answer
579.6k+ views
Hint: Hammond stated that if a transition state and an unstable intermediate occur in consecutive manner, then they should have relatively the same energy content. Because of this, the interconversion will involve small rearrangement of structures.
Complete answer:
Here, we are given an energy profile diagram for a two step reaction. We need to find how the transition states of the exothermic reaction would look like.
- Hammond’s postulate is a hypothesis in chemistry. It gives information about the geometric structure of the transition state of the given organic reaction. Thus, we can predict the geometric structure of a state by comparing its energy with neighboring species in the reaction coordinate.
- He stated that if a transition state and an unstable intermediate occur in consecutive manner, then they should have relatively the same energy content. Because of this, the interconversion will involve small rearrangement of structures.
- Here, we are given that the reaction is an exothermic reaction. So, heat will be generated during the reaction.
- So, in an exothermic reaction, the transition state’s energy is more close to the energy value of reactants than the energy of products. So, we can say that the transition state will be more similar geometrically to the reactants than the products. In the graph, we can say that the early transition is closer to the reactant. So, it will be more reactant like.
Thus, we can conclude that in exothermic reactions, early transition states are reactant-like.
So, the correct answer is (A).
Note:
Note that if the reaction is endothermic, then the energy will be required from outside. Here, the transition state is closer in energy of the products than the energy of the reactants. Thus, in the case of endothermic reactions, the structure of transition state is more similar to the products than the reactants.
Complete answer:
Here, we are given an energy profile diagram for a two step reaction. We need to find how the transition states of the exothermic reaction would look like.
- Hammond’s postulate is a hypothesis in chemistry. It gives information about the geometric structure of the transition state of the given organic reaction. Thus, we can predict the geometric structure of a state by comparing its energy with neighboring species in the reaction coordinate.
- He stated that if a transition state and an unstable intermediate occur in consecutive manner, then they should have relatively the same energy content. Because of this, the interconversion will involve small rearrangement of structures.
- Here, we are given that the reaction is an exothermic reaction. So, heat will be generated during the reaction.
- So, in an exothermic reaction, the transition state’s energy is more close to the energy value of reactants than the energy of products. So, we can say that the transition state will be more similar geometrically to the reactants than the products. In the graph, we can say that the early transition is closer to the reactant. So, it will be more reactant like.
Thus, we can conclude that in exothermic reactions, early transition states are reactant-like.
So, the correct answer is (A).
Note:
Note that if the reaction is endothermic, then the energy will be required from outside. Here, the transition state is closer in energy of the products than the energy of the reactants. Thus, in the case of endothermic reactions, the structure of transition state is more similar to the products than the reactants.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




