
Consider the following reaction sequence:
$\text{ Metal (M) }\xrightarrow{\text{Very dil}\text{.HN}{{\text{O}}_{\text{3}}}}\text{ no reaction }$
$\text{ Metal (M) }\xrightarrow{\text{Conc}\text{.HN}{{\text{O}}_{\text{3}}}}\text{ light blue solution (A) + gas(B) }$
The incorrect statement(s) is (are):
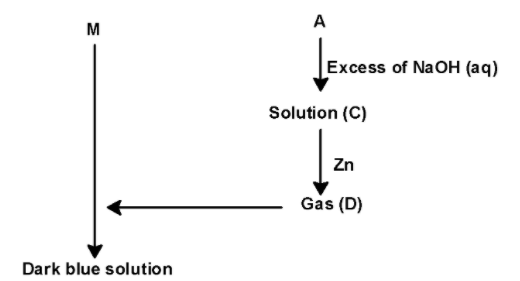
A) Gas (B) is diamagnetic.
B) Solution (C) contains the only $\text{ NaN}{{\text{O}}_{\text{2 }}}$ salt
C) Dark blue solution is paramagnetic
D) Metal (M) is extracted by the thermite process

Answer
576.6k+ views
Hint: nitric acid is an oxidizing agent. It oxidizes the metal M to $\text{ }{{\text{M}}^{\text{n+}}}\text{ }$. The nitric acid abstract the n electrons from the metal and reduced itself into the $\text{ N}{{\text{O}}_{\text{x}}}\text{ }$ gas and metal its nitrate salt. The metal can undergo the coordination complex interaction with the water. This gives a colour solution. Excess of sodium hydroxide in the solution forms a mixture of salts of nitrite and nitrate.
Complete step by step answer:
We have given the following reaction. Metal M does not react with dilute nitric acid $\text{ HN}{{\text{O}}_{\text{3 }}}$ .the general reaction is as represented below,
$\text{ Metal (M) }\xrightarrow{\text{Very dil}\text{.HN}{{\text{O}}_{\text{3}}}}\text{ no reaction }$
The metal reacts with the concentrated nitric acid and forms a blue coloured solution A with the liberation of gas B. The reaction is written as follows,
$\text{ Metal (M) }\xrightarrow{\text{Conc}\text{.HN}{{\text{O}}_{\text{3}}}}\text{ light blue solution (A) + gas(B) }$
Nitric acid is a strong oxidizing agent. It is used in a redox reaction.
Copper reacts with a concentrated nitric acid. Nitric acid itself accepts the electron and undergoes the reduction. Copper loses its two electrons and becomes $\text{ C}{{\text{u}}^{\text{2+}}}\text{ }$ .the oxidation reaction of copper is as shown below,
$\text{ Cu }\to \text{ C}{{\text{u}}^{\text{2+}}}\text{ + 2}{{\text{e}}^{-}}\text{ }$
The nitric acid accepts these two electrons and forms a nitrate ion. The reduction reaction of nitric acid is as shown below,
$\text{ 4HN}{{\text{O}}_{\text{3}}}\text{ + 2}{{\text{e}}^{-}}\text{ }\to \text{ 2NO}_{3}^{-}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O }$
The reaction of copper with the concentrated nitric acid is as shown below,
$\text{ 4HN}{{\text{O}}_{\text{3}}}\text{ + Cu }\to \text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ + 2N}{{\text{O}}_{\text{2}}}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O }$
The speed of the reaction depends on the surface area of copper.
The solution changes the colour because it is an aqueous solution. The copper ion undergoes the coordination of complex interactions with the surrounding water molecules. This cording complex with the water is blue in colour.
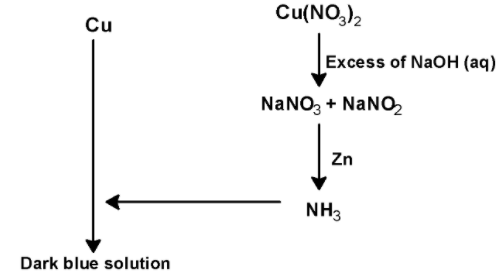
This nitrogen dioxide $\text{ N}{{\text{O}}_{\text{2}}}\text{ }$ is paramagnetic in nature. The nitrogen has an unpaired electron which makes it paramagnetic. Copper cannot be extracted through the thermite process. When copper nitrate solution is treated with sodium hydroxide$\text{ NaOH }$ , the copper hydroxide is formed as the product. The reaction of $\text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }$ with $\text{ NaOH }$as shown below,
$\text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }+\text{ 2NaOH }\to \text{ Cu(OH}{{\text{)}}_{\text{2}}}\text{ + 2NaN}{{\text{O}}_{\text{3}}}$
The solution also contains $\text{ NaN}{{\text{O}}_{\text{2}}}\text{ }$ .solution Cis a mixture of$\text{ NaN}{{\text{O}}_{3}}\text{ + NaN}{{\text{O}}_{\text{2}}}\text{ }$. This solution C when treated with ammonia liberates ammonia gas.
Thus, in the given reaction
Metal (M) is $\text{ Cu }$ ,
Blue solution (A) is $\text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }$ ,
Gas (B) is$\text{ N}{{\text{O}}_{\text{2}}}\text{ }$. It is paramagnetic.
Solution C is $\text{ NaN}{{\text{O}}_{3}}\text{ + NaN}{{\text{O}}_{\text{2}}}\text{ }$
Gas (D) is $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$
Thus, statement (C) correctly is valid.
Hence, (A), (B) and (D) are correct options.
Note: Dilute nitric acid reacts with the copper. However, the reaction for nitrogen oxide is the by -product. The nitric acid and oxidizing agent thus favours the reaction. Mineral acid like hydrochloric acid does not oxidize the copper, but sulphuric acid is a strong oxidizing agent and reacts with copper to release sulphur dioxide as a gas.
Complete step by step answer:
We have given the following reaction. Metal M does not react with dilute nitric acid $\text{ HN}{{\text{O}}_{\text{3 }}}$ .the general reaction is as represented below,
$\text{ Metal (M) }\xrightarrow{\text{Very dil}\text{.HN}{{\text{O}}_{\text{3}}}}\text{ no reaction }$
The metal reacts with the concentrated nitric acid and forms a blue coloured solution A with the liberation of gas B. The reaction is written as follows,
$\text{ Metal (M) }\xrightarrow{\text{Conc}\text{.HN}{{\text{O}}_{\text{3}}}}\text{ light blue solution (A) + gas(B) }$
Nitric acid is a strong oxidizing agent. It is used in a redox reaction.
Copper reacts with a concentrated nitric acid. Nitric acid itself accepts the electron and undergoes the reduction. Copper loses its two electrons and becomes $\text{ C}{{\text{u}}^{\text{2+}}}\text{ }$ .the oxidation reaction of copper is as shown below,
$\text{ Cu }\to \text{ C}{{\text{u}}^{\text{2+}}}\text{ + 2}{{\text{e}}^{-}}\text{ }$
The nitric acid accepts these two electrons and forms a nitrate ion. The reduction reaction of nitric acid is as shown below,
$\text{ 4HN}{{\text{O}}_{\text{3}}}\text{ + 2}{{\text{e}}^{-}}\text{ }\to \text{ 2NO}_{3}^{-}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O }$
The reaction of copper with the concentrated nitric acid is as shown below,
$\text{ 4HN}{{\text{O}}_{\text{3}}}\text{ + Cu }\to \text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ + 2N}{{\text{O}}_{\text{2}}}\text{ + 2}{{\text{H}}_{\text{2}}}\text{O }$
The speed of the reaction depends on the surface area of copper.
The solution changes the colour because it is an aqueous solution. The copper ion undergoes the coordination of complex interactions with the surrounding water molecules. This cording complex with the water is blue in colour.
This nitrogen dioxide $\text{ N}{{\text{O}}_{\text{2}}}\text{ }$ is paramagnetic in nature. The nitrogen has an unpaired electron which makes it paramagnetic. Copper cannot be extracted through the thermite process. When copper nitrate solution is treated with sodium hydroxide$\text{ NaOH }$ , the copper hydroxide is formed as the product. The reaction of $\text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }$ with $\text{ NaOH }$as shown below,
$\text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }+\text{ 2NaOH }\to \text{ Cu(OH}{{\text{)}}_{\text{2}}}\text{ + 2NaN}{{\text{O}}_{\text{3}}}$
The solution also contains $\text{ NaN}{{\text{O}}_{\text{2}}}\text{ }$ .solution Cis a mixture of$\text{ NaN}{{\text{O}}_{3}}\text{ + NaN}{{\text{O}}_{\text{2}}}\text{ }$. This solution C when treated with ammonia liberates ammonia gas.
Thus, in the given reaction
Metal (M) is $\text{ Cu }$ ,
Blue solution (A) is $\text{ Cu(N}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{ }$ ,
Gas (B) is$\text{ N}{{\text{O}}_{\text{2}}}\text{ }$. It is paramagnetic.
Solution C is $\text{ NaN}{{\text{O}}_{3}}\text{ + NaN}{{\text{O}}_{\text{2}}}\text{ }$
Gas (D) is $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$

Thus, statement (C) correctly is valid.
Hence, (A), (B) and (D) are correct options.
Note: Dilute nitric acid reacts with the copper. However, the reaction for nitrogen oxide is the by -product. The nitric acid and oxidizing agent thus favours the reaction. Mineral acid like hydrochloric acid does not oxidize the copper, but sulphuric acid is a strong oxidizing agent and reacts with copper to release sulphur dioxide as a gas.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




