
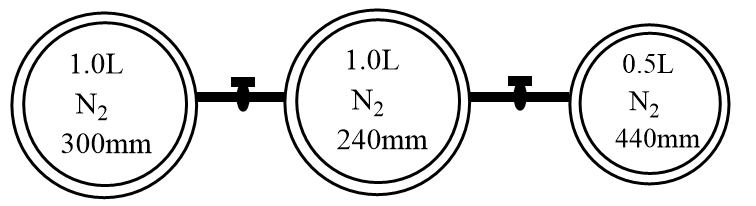
Consider the bulbs shown in the arrangement below. If the pressure of the system when all the stop cocks are opened is $x$ (in atm) then find $100x$. ($760\;{\text{mm}} = 1\;{\text{atm}}$)

Answer
508.5k+ views
Hint: Boyle’s law which is an experimental gas law which states that the pressure of a gas is inversely proportional to its volume when the number of moles of gas and temperature of the system is considered as constant. In simple words, it describes how pressure of the gas tends to decrease in increasing the volume of the container for an isothermal process.
Complete answer:
For comparing the same gases under two different sets of conditions, the Boyle’s law is expressed as follows:
${P_1}{V_1} = {P_2}{V_2}$
Where, ${P_1}$ and ${V_1}$ are the pressure and volume of gas at initial conditions while ${P_2}$ and ${V_2}$ are the pressure and volume of gas in final conditions.
When two or more moles of gases are mixed, then the Boyle’s will be represented as follows:
$PV = {P_1}{V_1} + {P_2}{V_2}$
Where P is the pressure of the gas after mixing and V is the final volume of the container which is equal to the sum of the initial volumes i.e., $V = {V_1} + {V_2}$.
For the given case, the nitrogen gas is present under a different set of conditions in three bulbs. When the stop cork is opened, the gases will start mixing. Assuming the system to be isothermal and ideal mixing of gases, the final volume and pressure can be represented by the following expression:
$PV = {P_1}{V_1} + {P_2}{V_2} + {P_3}{V_3}$
$ \Rightarrow P({V_1} + {V_2} + {V_3}) = {P_1}{V_1} + {P_2}{V_2} + {P_3}{V_3}$
Substituting given values:
\[ \Rightarrow x(1 + 1 + 0.5) = 300 \times 1 + 240 \times 1 + 440 \times 0.5\]
$ \Rightarrow 2.5x = 760$
$ \Rightarrow x = 304\;mm$
Now, it is given in the question that $760\;{\text{mm}} = 1\;{\text{atm}}$. So, converting pressure in unit of atm, the value of x will be as follows:
$ \Rightarrow x = \dfrac{{304}}{{760}}$
$ \Rightarrow x = 0.4\;{\text{atm}}$
Therefore, the value of $100x$ will be as follows:
$100x = 100 \times 0.4$
$ \Rightarrow 100x = 40\;{\text{atm}}$
Hence, as per given conditions the value of $100x$ is $40\;{\text{atm}}$.
Note:
It is important to note that we can alternatively calculate the final pressure of the system by calculating partial pressure of pressure of each set of nitrogen at different condition using ideal gas equation and then according to Dalton’s law of partial pressure, the total pressure of the gas will be equal to the summation of calculated partial pressures.
Complete answer:
For comparing the same gases under two different sets of conditions, the Boyle’s law is expressed as follows:
${P_1}{V_1} = {P_2}{V_2}$
Where, ${P_1}$ and ${V_1}$ are the pressure and volume of gas at initial conditions while ${P_2}$ and ${V_2}$ are the pressure and volume of gas in final conditions.
When two or more moles of gases are mixed, then the Boyle’s will be represented as follows:
$PV = {P_1}{V_1} + {P_2}{V_2}$
Where P is the pressure of the gas after mixing and V is the final volume of the container which is equal to the sum of the initial volumes i.e., $V = {V_1} + {V_2}$.
For the given case, the nitrogen gas is present under a different set of conditions in three bulbs. When the stop cork is opened, the gases will start mixing. Assuming the system to be isothermal and ideal mixing of gases, the final volume and pressure can be represented by the following expression:
$PV = {P_1}{V_1} + {P_2}{V_2} + {P_3}{V_3}$
$ \Rightarrow P({V_1} + {V_2} + {V_3}) = {P_1}{V_1} + {P_2}{V_2} + {P_3}{V_3}$
Substituting given values:
\[ \Rightarrow x(1 + 1 + 0.5) = 300 \times 1 + 240 \times 1 + 440 \times 0.5\]
$ \Rightarrow 2.5x = 760$
$ \Rightarrow x = 304\;mm$
Now, it is given in the question that $760\;{\text{mm}} = 1\;{\text{atm}}$. So, converting pressure in unit of atm, the value of x will be as follows:
$ \Rightarrow x = \dfrac{{304}}{{760}}$
$ \Rightarrow x = 0.4\;{\text{atm}}$
Therefore, the value of $100x$ will be as follows:
$100x = 100 \times 0.4$
$ \Rightarrow 100x = 40\;{\text{atm}}$
Hence, as per given conditions the value of $100x$ is $40\;{\text{atm}}$.
Note:
It is important to note that we can alternatively calculate the final pressure of the system by calculating partial pressure of pressure of each set of nitrogen at different condition using ideal gas equation and then according to Dalton’s law of partial pressure, the total pressure of the gas will be equal to the summation of calculated partial pressures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




