
What is the concentration or enrichment of ore? Explain the method of enrichment of ore containing sulphide with diagram?
Answer
596.1k+ views
Hint:
We know that minerals are present in ores. The solid materials from which a pure metal can be obtained is called ore. The minerals in the ore are present with unwanted minerals called impurities.
Complete step by step solution:
As we know that minerals are present in ores. To extract minerals we have to dig the ore of that mineral. Minerals are present with some unwanted materials called impurities. To remove these unwanted substances the ores are concentrated on the basic of the type of impurities and their percentage proportion. There are different procedures for the concentration of ores such as hydraulic washing, magnetic separation, froth floatation process, leaching etc.
- Froth flotation method is used for enrichment of ore containing sulphide.
- The term flotation refers to separating mineral particles according to the difference in their physical and chemical properties.
- This method is particularly used for sulphide minerals as sulphide minerals have high float ability.
- In this method of froth flotation water and fine powder of the ore are filled in a big vessel. After this substances like pine, or turpentine oil is poured into it. By doing so the sulphides of metal get wet and stick to it, while clay and particles of sand do not get wet.
- After this air is passed with pressure through a tube.
- Froth is produced along with the light particles of the sulphide ore and comes to the surface of the liquid mixture. Heavy particles become wet and settle down at the bottom.
- Now the sulphide ore is removed with sieves ( a utensil used for straining solid from liquid ) in other vessel and washed with water.
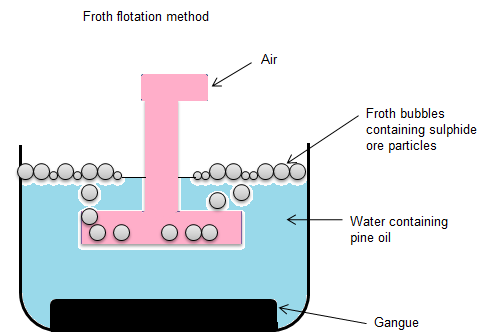
Gangue also means impurities.
Note:
Remember that the process of froth flotation is mainly used for sulphide ores of copper, lead and zinc. Apart from these it can also be used for the concentration of Cinnabar ( HgS ) the ore of mercury.
We know that minerals are present in ores. The solid materials from which a pure metal can be obtained is called ore. The minerals in the ore are present with unwanted minerals called impurities.
Complete step by step solution:
As we know that minerals are present in ores. To extract minerals we have to dig the ore of that mineral. Minerals are present with some unwanted materials called impurities. To remove these unwanted substances the ores are concentrated on the basic of the type of impurities and their percentage proportion. There are different procedures for the concentration of ores such as hydraulic washing, magnetic separation, froth floatation process, leaching etc.
- Froth flotation method is used for enrichment of ore containing sulphide.
- The term flotation refers to separating mineral particles according to the difference in their physical and chemical properties.
- This method is particularly used for sulphide minerals as sulphide minerals have high float ability.
- In this method of froth flotation water and fine powder of the ore are filled in a big vessel. After this substances like pine, or turpentine oil is poured into it. By doing so the sulphides of metal get wet and stick to it, while clay and particles of sand do not get wet.
- After this air is passed with pressure through a tube.
- Froth is produced along with the light particles of the sulphide ore and comes to the surface of the liquid mixture. Heavy particles become wet and settle down at the bottom.
- Now the sulphide ore is removed with sieves ( a utensil used for straining solid from liquid ) in other vessel and washed with water.

Gangue also means impurities.
Note:
Remember that the process of froth flotation is mainly used for sulphide ores of copper, lead and zinc. Apart from these it can also be used for the concentration of Cinnabar ( HgS ) the ore of mercury.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




