
Complete the following reactions?
1. $ N{a_2}O + {H_2}O \to \underline {} \underline {} \underline {} \underline {} \underline {} $ ?
2. $ \underline {} \underline {} \underline {} \underline {} + \underline {} \underline {} \underline {} \underline {} \to {H_2}C{O_2} $ ?
Answer
495.3k+ views
Hint: The substances which participate and chemically change during the reaction are known as reactants whereas those substances which are produced during the chemical reaction are known as products. The representation of a chemical reaction is a chemical equation.
Complete answer:
We will solve it in two steps. The first step includes the reaction of sodium oxide with water. Here Sodium Oxide $ (N{a_2}O) $ is a metal oxide which crystallizes in the antifluorite structure. Second step includes the formation of formic acid $ ({H_2}C{O_2}) $ which we can obtain by the process of hydrolysis.
Step-1: $ 1.N{a_2}O + {H_2}O \to \underline {} \underline {} \underline {} \underline {} \underline {} $
As we know, Sodium Oxide is a compound whose chemical formula is $ N{a_2}O $ . It is used in glasses and ceramics. When this compound reacts with water $ {H_2}O $ , it gives sodium hydroxide. This compound is the basic anhydride of sodium hydroxide.
The complete chemical reaction is:
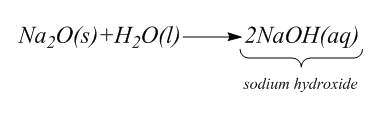
Step-2: $ 2.\underline {} \underline {} \underline {} \underline {} \underline {} + \underline {} \underline {} \underline {} \underline {} \underline {} \to {H_2}C{O_2} $
For this part we have to synthesize formic acid $ ({H_2}C{O_2}) $ . For this, first we will use carbonylation of methyl alcohol to form esters.
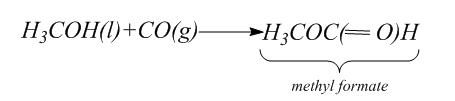
Then we will hydrolyze the methyl formate to form the formic acid and methanol. Hydrolysis is the process in which the bond between the elements of the compound break. Thus, in hydrolysis, addition of water breaks one or two chemical bonds.
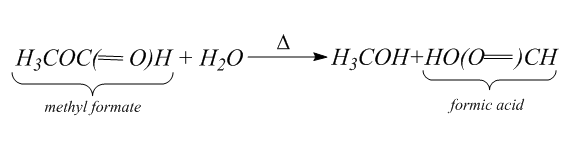
Hydrolysis is the reverse of the condensation which is the process of joining two molecules into a larger one and eliminates a water molecule.
Note:
Formic acid is also formed by the reaction of methyl formate with ammonia. Hydrolysis of methyl formate required a huge excess of water so some routes use the reaction of ammonia with methyl formate to form formamide which on hydrolysis with sulfuric acid gives formic acid.
Complete answer:
We will solve it in two steps. The first step includes the reaction of sodium oxide with water. Here Sodium Oxide $ (N{a_2}O) $ is a metal oxide which crystallizes in the antifluorite structure. Second step includes the formation of formic acid $ ({H_2}C{O_2}) $ which we can obtain by the process of hydrolysis.
Step-1: $ 1.N{a_2}O + {H_2}O \to \underline {} \underline {} \underline {} \underline {} \underline {} $
As we know, Sodium Oxide is a compound whose chemical formula is $ N{a_2}O $ . It is used in glasses and ceramics. When this compound reacts with water $ {H_2}O $ , it gives sodium hydroxide. This compound is the basic anhydride of sodium hydroxide.
The complete chemical reaction is:
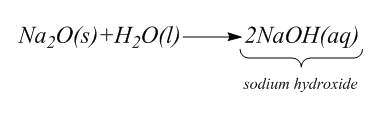
Step-2: $ 2.\underline {} \underline {} \underline {} \underline {} \underline {} + \underline {} \underline {} \underline {} \underline {} \underline {} \to {H_2}C{O_2} $
For this part we have to synthesize formic acid $ ({H_2}C{O_2}) $ . For this, first we will use carbonylation of methyl alcohol to form esters.
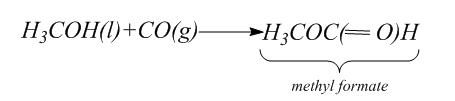
Then we will hydrolyze the methyl formate to form the formic acid and methanol. Hydrolysis is the process in which the bond between the elements of the compound break. Thus, in hydrolysis, addition of water breaks one or two chemical bonds.
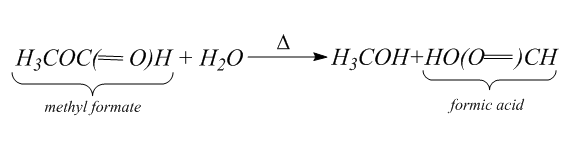
Hydrolysis is the reverse of the condensation which is the process of joining two molecules into a larger one and eliminates a water molecule.
Note:
Formic acid is also formed by the reaction of methyl formate with ammonia. Hydrolysis of methyl formate required a huge excess of water so some routes use the reaction of ammonia with methyl formate to form formamide which on hydrolysis with sulfuric acid gives formic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




