
Complete the following reaction:
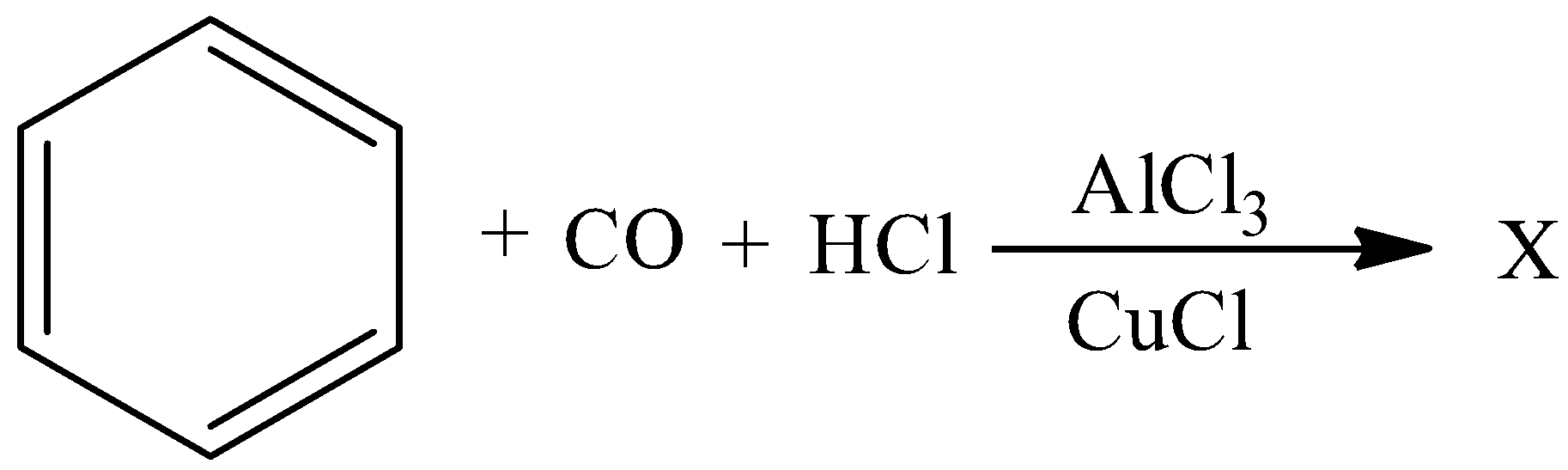
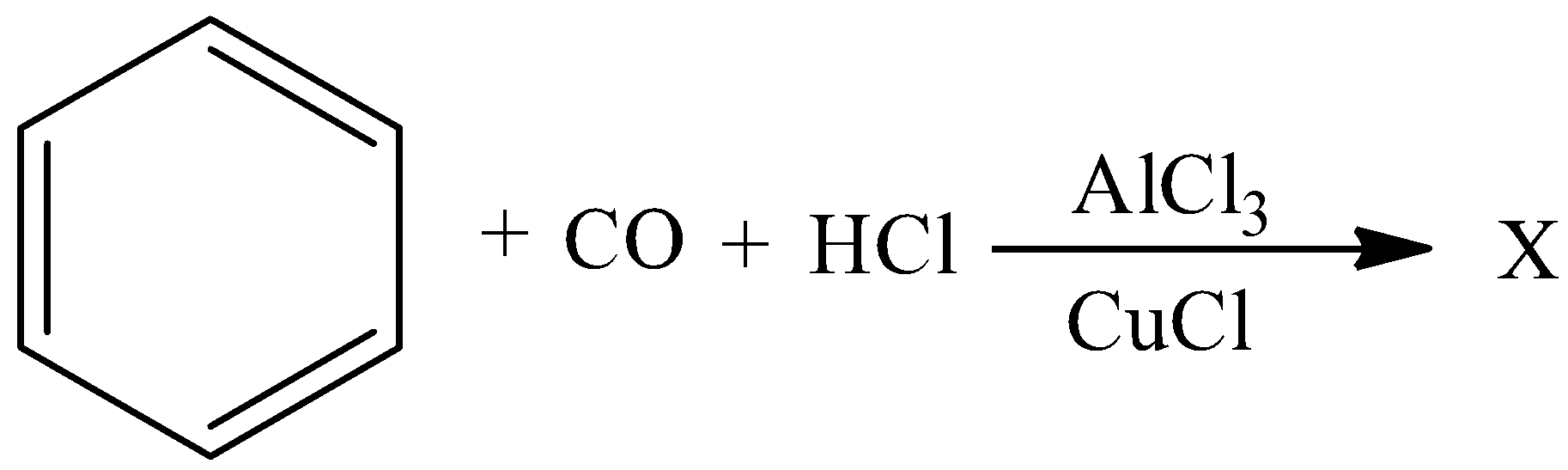
Answer
515.1k+ views
Hint: In this reaction we use gattermann Koch formylation. The Gattermann reaction, is a chemical reaction in which aromatic compounds are formulated by a mixture of hydrogen cyanide and hydrogen chloride in the presence of a Lewis acid catalyst such as $AlC{l_3}$.
Complete answer: Gattermann Koch formylation is the preparation of aromatic aldehydes by the treatment of aromatic compounds with CO and HCl in the presence of aluminium chloride and cuprous chloride, by which carbon monoxide and hydrochloric acid behave like the formyl chloride and react in a fashion similar to other acyl chlorides. This electrophilic aromatic substitution allows the synthesis of monoacetylated products from the reaction between arenes and acyl chlorides or anhydrides.
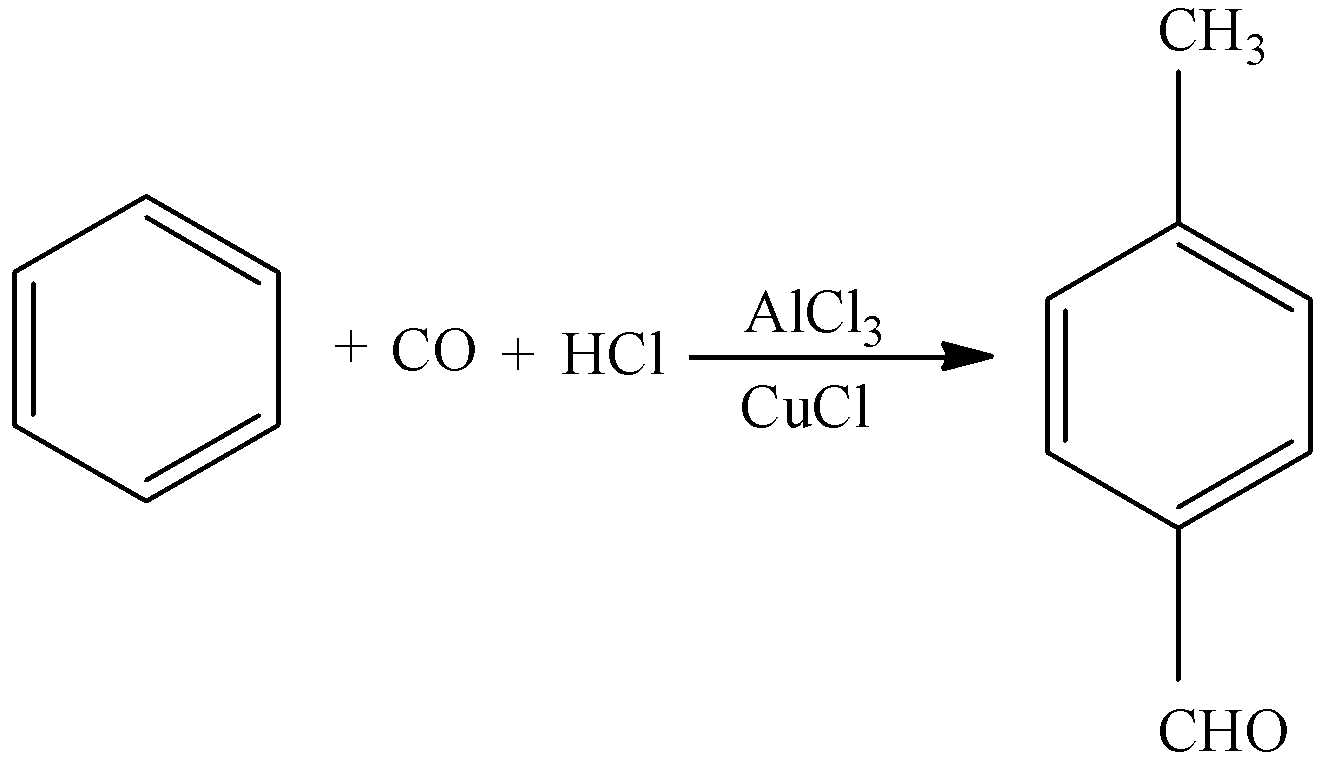
In this reaction benzene reacts with carbon monoxide and hydrochloric acid in the presence of aluminium chloride and copper chloride to give $4 - $methylbenzaldehyde. It is a colorless liquid. Commercially available and has a cherry like scent similar to benzaldehyde.
Additional information:
Aluminum chloride is widely used in petroleum refining and the manufacturing of many products. In addition, antiperspirants and cosmetic astringents. It is also used in the production of aluminium metal, but it also has a wide number of uses in the chemical industry particularly as a Lewis acid. Solid aluminium chloride is covalently bonded with low melting as well as boiling point.
Note:
In this reaction the highly unstable formyl chloride was initially postulated as an intermediate, formyl cation, is now thought to react directly with the arena without the initial formation of formyl chloride. When the carbon monoxide is not used at high pressure, the presence of traces of copper chloride is often necessary.
Complete answer: Gattermann Koch formylation is the preparation of aromatic aldehydes by the treatment of aromatic compounds with CO and HCl in the presence of aluminium chloride and cuprous chloride, by which carbon monoxide and hydrochloric acid behave like the formyl chloride and react in a fashion similar to other acyl chlorides. This electrophilic aromatic substitution allows the synthesis of monoacetylated products from the reaction between arenes and acyl chlorides or anhydrides.

In this reaction benzene reacts with carbon monoxide and hydrochloric acid in the presence of aluminium chloride and copper chloride to give $4 - $methylbenzaldehyde. It is a colorless liquid. Commercially available and has a cherry like scent similar to benzaldehyde.
Additional information:
Aluminum chloride is widely used in petroleum refining and the manufacturing of many products. In addition, antiperspirants and cosmetic astringents. It is also used in the production of aluminium metal, but it also has a wide number of uses in the chemical industry particularly as a Lewis acid. Solid aluminium chloride is covalently bonded with low melting as well as boiling point.
Note:
In this reaction the highly unstable formyl chloride was initially postulated as an intermediate, formyl cation, is now thought to react directly with the arena without the initial formation of formyl chloride. When the carbon monoxide is not used at high pressure, the presence of traces of copper chloride is often necessary.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




