
Complete the following reaction:
$ 2C{{H}_{2}}=O\xrightarrow{ConcKOH} $
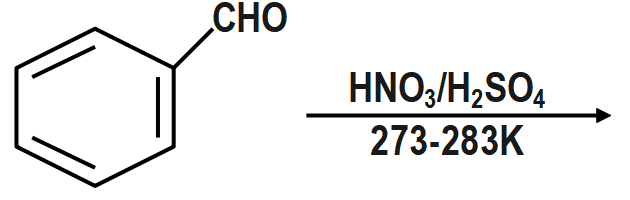
$ C{{H}_{3}}COOH\xrightarrow{B{{r}_{2}}/P} $

Answer
528.9k+ views
Hint :We know that to complete the above reaction we have to know the Cannizaro reaction and Hell-Volhard reaction. The cannizzaro reaction was named after the discoverer named Stanislao Cannizaro. In this reaction the formation of the primary alcohol and the carboxylic acid takes place. The Cannizaro is a type of redox reaction.
Complete Step By Step Answer:
As the cannizaro is a redox reaction in which the aldehyde molecules which are two in number are reacted using the hydroxide base to form the product as primary alcohol and the carboxylic acid. In the Cannizaro reaction firstly we see that the hydroxide tends to attack the carbonyl carbon which further undergoes deprotonation to form dianion.this unstable intermediate compound which is formed tends to release hydride anion which tends to attack the another molecule of the aldehyde. During this process the dianion which is formed tends to convert the carboxylate anion and the aldehyde into the alkoxide. This alkoxide formed in the reaction then picks up the proton from the water that produces the alcohol as the final product whereas the carboxylate is converted into the carboxylic acid product after the acid works up. The product in the cannizaro reaction is the alcohol and the salt of the corresponding acid.
So when the two molecules of formaldehyde are being reacted under concentrated potassium hydroxide the formation of methanol and the potassium formate. The reaction is as follows:
$ 2C{{H}_{2}}=O\xrightarrow{ConcKOH}\left[ C{{H}_{3}}-OH \right]+\left[ O=CH-OK \right] $
Similarly, $ C{{H}_{3}}COOH\xrightarrow{B{{r}_{2}}/P}Br-C{{H}_{2}}-COOH $ and
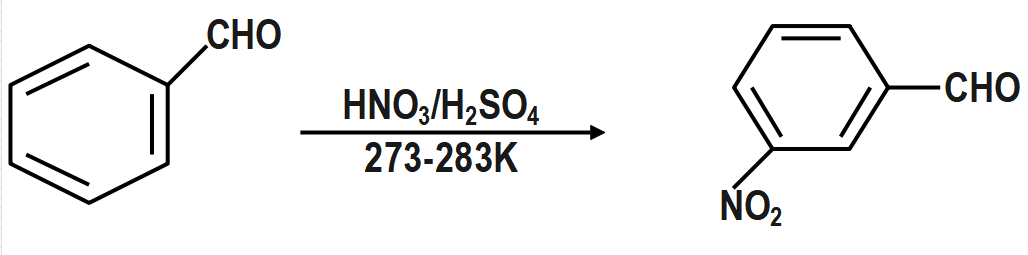
Note :
Note that in the cross cannizzaro reaction we see that one of the molecules of the aldehyde gets oxidised whereas the other gets reduced. The one mol of the aldehyde gives us the alcohol and the other mole gives us carboxylic acid in cross cannizzaro reaction. Here the two molecules of the aldehyde are different. The aldehyde in cross cannizzaro does not have alpha hydrogen present in it.
Complete Step By Step Answer:
As the cannizaro is a redox reaction in which the aldehyde molecules which are two in number are reacted using the hydroxide base to form the product as primary alcohol and the carboxylic acid. In the Cannizaro reaction firstly we see that the hydroxide tends to attack the carbonyl carbon which further undergoes deprotonation to form dianion.this unstable intermediate compound which is formed tends to release hydride anion which tends to attack the another molecule of the aldehyde. During this process the dianion which is formed tends to convert the carboxylate anion and the aldehyde into the alkoxide. This alkoxide formed in the reaction then picks up the proton from the water that produces the alcohol as the final product whereas the carboxylate is converted into the carboxylic acid product after the acid works up. The product in the cannizaro reaction is the alcohol and the salt of the corresponding acid.
So when the two molecules of formaldehyde are being reacted under concentrated potassium hydroxide the formation of methanol and the potassium formate. The reaction is as follows:
$ 2C{{H}_{2}}=O\xrightarrow{ConcKOH}\left[ C{{H}_{3}}-OH \right]+\left[ O=CH-OK \right] $
Similarly, $ C{{H}_{3}}COOH\xrightarrow{B{{r}_{2}}/P}Br-C{{H}_{2}}-COOH $ and
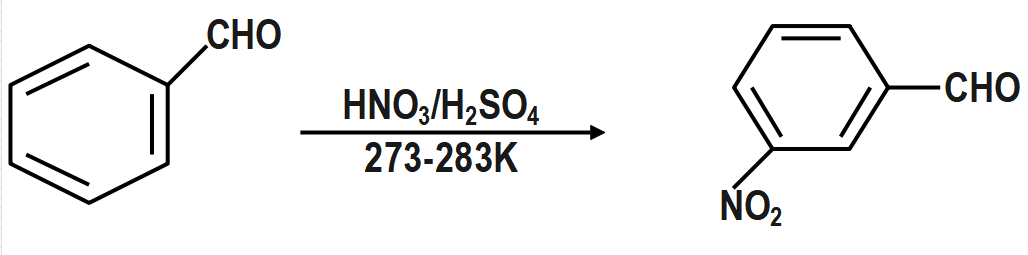
Note :
Note that in the cross cannizzaro reaction we see that one of the molecules of the aldehyde gets oxidised whereas the other gets reduced. The one mol of the aldehyde gives us the alcohol and the other mole gives us carboxylic acid in cross cannizzaro reaction. Here the two molecules of the aldehyde are different. The aldehyde in cross cannizzaro does not have alpha hydrogen present in it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




