
Complete the following reaction:
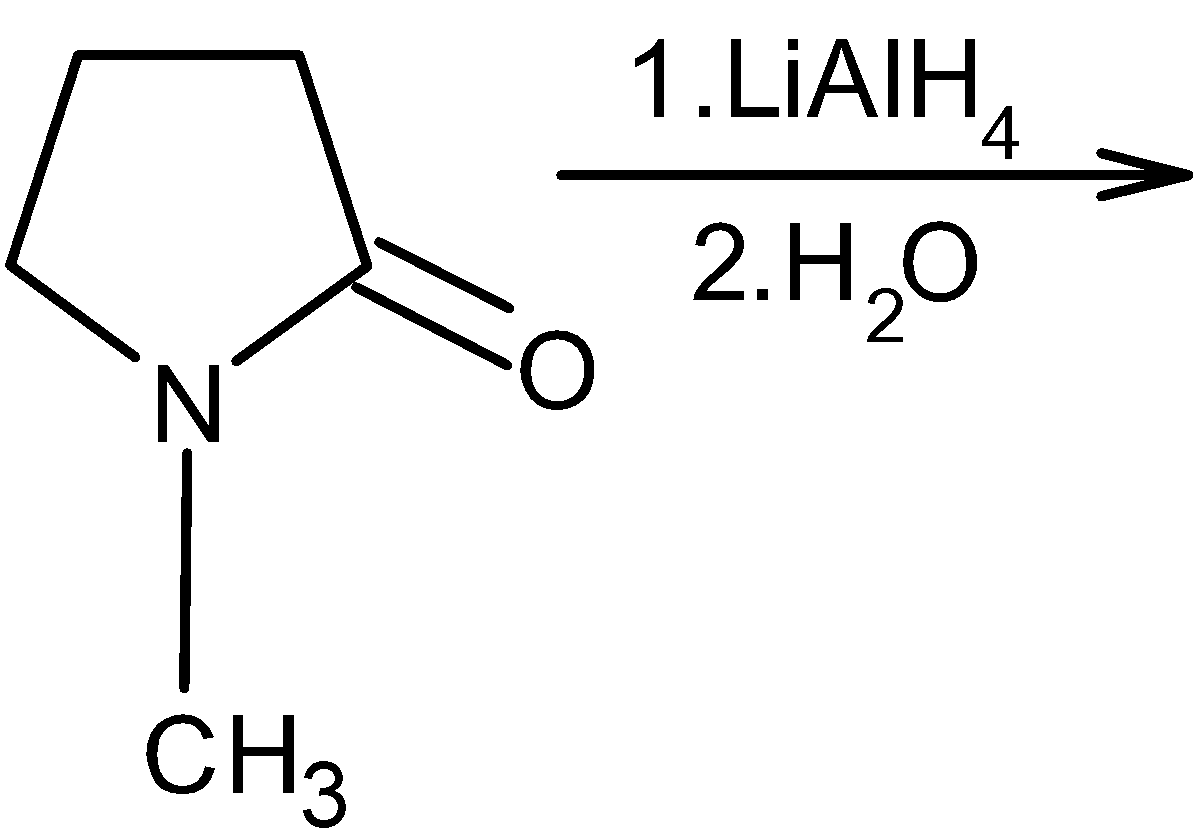

Answer
586.2k+ views
Hint: The lithium aluminium hydride is a reducing agent. The reduction process involves the addition of a hydrogen atom or removal of an oxygen atom. The LAH reduces the aldehyde and ketones into the alcohol. The amides are reduced differently in the presence of LAH. The lone pair on the nitrogen assist the reduction of the carbonyl group.
Complete step by step reaction:
The lithium aluminium hydride is a widely used reducing agent. It has 4 hydrogen atoms. It adds the hydrogen atom to the organic compound and reduces it. The carbonyl groups (in aldehyde and ketone) are generally reduced to the primary and secondary alcohol.
However, the LAH reduces the amides group to the amine. The lone pair of the nitrogen assists the mechanism. The lone pair pushes or knocks out the oxygen. The oxygen is better leaving the group than nitrogen.
The reaction mechanism involves the following steps:
Step 1) The LAH attacks on the carbonyl carbon, such that the LAH transfers its proton to the carbonyl atom, and the oxygen acquires the lone pair of electrons.
Step 2) The lone pair on the electrons shift to the adjacent carbon atom followed by the removal of the oxygen atom. The nitrogen acquires a positive charge. The resonating structures are drawn to show the removal of the oxygen atom.
Step 3) In the next step, the next molecule of LAH attacks on the carbon followed by nitrogen accommodating its lone pair.
Thereby, the amide is converted into an amine.
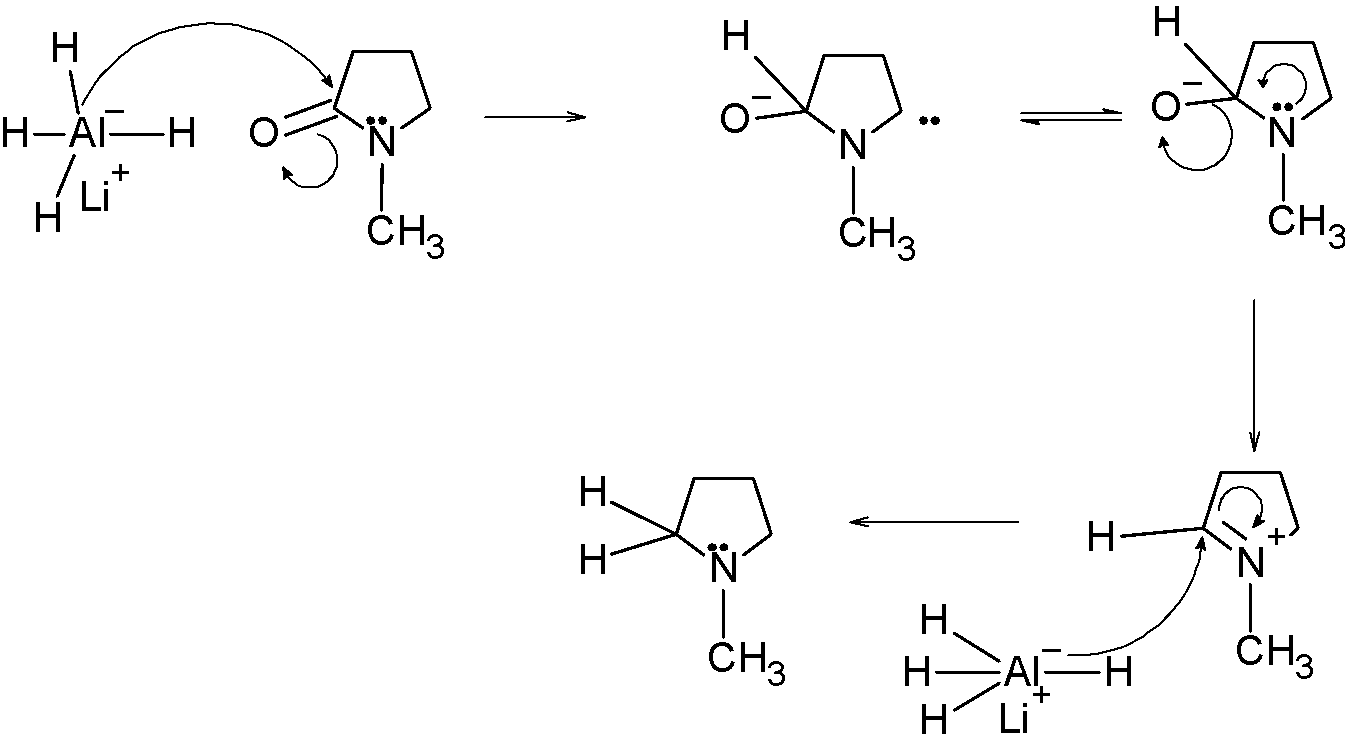
Hence, the obtained product is N-methyl pyrrolidine.
Additional Information:
Among the complex hydrides, Lithium Aluminium Hydride is the strongest reducing agent because it reduces four molecules at a time.
Complex hydrides in general cannot reduce the non-polar carbon-carbon pi-bonds. That is, these reducing agents are not used in the reduction or hydrogenation of alkenes and alkynes.
Since Lithium Aluminium Hydride is a strong reducing agent, it can reduce carboxylic acids, esters, and amides.
Sodium borohydride cannot reduce an ester, an amide, or carboxylic acids. It can be used to selectively reduce an aldehyde or a ketone group in a compound.
Note: At a glance, it looks like the carbonyl group is reduced to the hydroxyl group. However, that cannot be true. The amide contains the lone pair of electrons on the nitrogen atom which pushes out the oxygen. But, in aldehyde or ketone , no atom contains the lone pair, thus the reduction ends at the hydroxyl group. Note that acid workup is used for the protonation, but it can be assumed as there is a tiresome step.
Complete step by step reaction:
The lithium aluminium hydride is a widely used reducing agent. It has 4 hydrogen atoms. It adds the hydrogen atom to the organic compound and reduces it. The carbonyl groups (in aldehyde and ketone) are generally reduced to the primary and secondary alcohol.
However, the LAH reduces the amides group to the amine. The lone pair of the nitrogen assists the mechanism. The lone pair pushes or knocks out the oxygen. The oxygen is better leaving the group than nitrogen.
The reaction mechanism involves the following steps:
Step 1) The LAH attacks on the carbonyl carbon, such that the LAH transfers its proton to the carbonyl atom, and the oxygen acquires the lone pair of electrons.
Step 2) The lone pair on the electrons shift to the adjacent carbon atom followed by the removal of the oxygen atom. The nitrogen acquires a positive charge. The resonating structures are drawn to show the removal of the oxygen atom.
Step 3) In the next step, the next molecule of LAH attacks on the carbon followed by nitrogen accommodating its lone pair.
Thereby, the amide is converted into an amine.

Hence, the obtained product is N-methyl pyrrolidine.
Additional Information:
Among the complex hydrides, Lithium Aluminium Hydride is the strongest reducing agent because it reduces four molecules at a time.
Complex hydrides in general cannot reduce the non-polar carbon-carbon pi-bonds. That is, these reducing agents are not used in the reduction or hydrogenation of alkenes and alkynes.
Since Lithium Aluminium Hydride is a strong reducing agent, it can reduce carboxylic acids, esters, and amides.
Sodium borohydride cannot reduce an ester, an amide, or carboxylic acids. It can be used to selectively reduce an aldehyde or a ketone group in a compound.
Note: At a glance, it looks like the carbonyl group is reduced to the hydroxyl group. However, that cannot be true. The amide contains the lone pair of electrons on the nitrogen atom which pushes out the oxygen. But, in aldehyde or ketone , no atom contains the lone pair, thus the reduction ends at the hydroxyl group. Note that acid workup is used for the protonation, but it can be assumed as there is a tiresome step.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




