
Complete the following reaction.
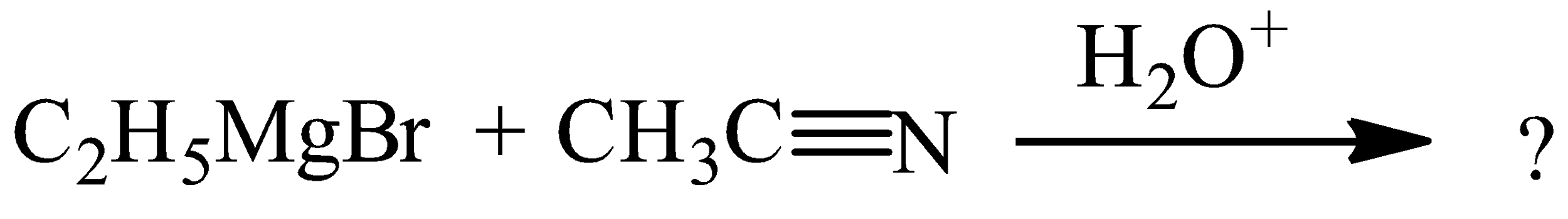
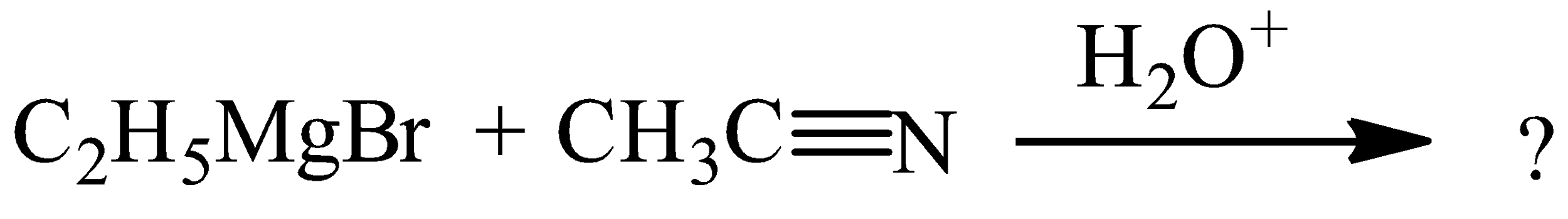
Answer
514.8k+ views
Hint: The Grignard Reaction is the addition of an organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively. The reaction with formaldehyde leads to a primary alcohol.
Complete answer: The Grignard reaction is an organometallic synthetic reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) is added to a carbonyl gathering in an aldehyde or ketone. This reaction is significant for the development of carbon–carbon bonds. The reaction of a natural halide with magnesium isn't a Grignard reaction, however it gives a Grignard reagent.
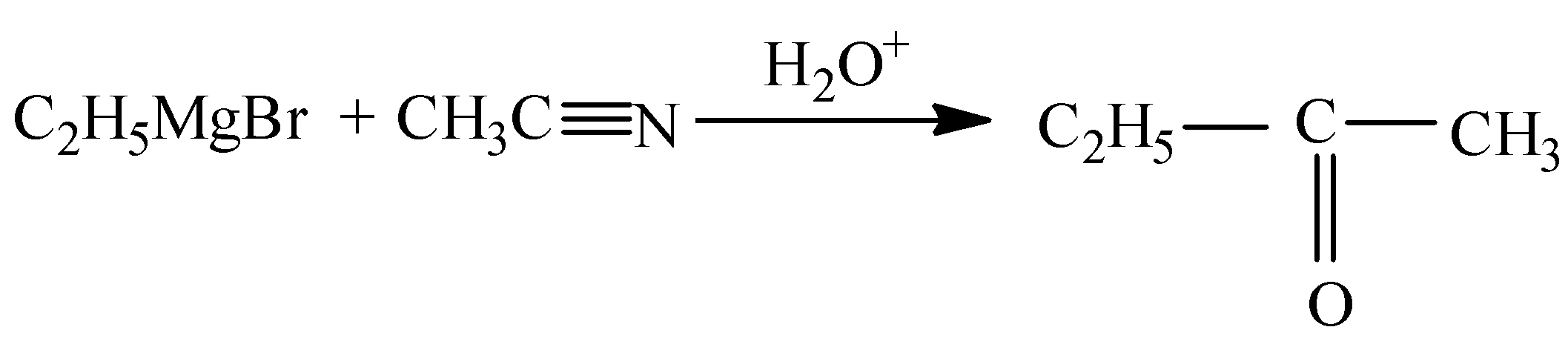
When ethylmagnesium bromide reacts with acetonitrile in the presence of oxonium it gives butanone.
Magnesium atom is used in the Grignard reagent because magnesium metal is fairly easily oxidized and the Grignard reagent, while water sensitive, is not so air sensitive, and magnesium metal is cheap, and easy to deploy.
Additional information:
Grignard reagents are made through the expansion of magnesium metal to alkyl or alkenyl halides. The halide can be $Cl,$$Br$,$I,$ (not $F$) . It's marginally simpler to make Grignards from the iodides and bromides, nonetheless. The magnesium is embedding itself between the carbon and the halide. This halides the $X$ alluded to when we allude to Grignard reagents as $RMgX$.They're very acceptable nucleophiles, responding with electrophiles like carbonyl mixtures and epoxides. They're additionally solid bases and will respond with acidic hydrogens.
Note:
Grignard reagents are utilized artificially to frame new carbon–carbon bonds. A Grignard reagent has a polar carbon–magnesium bond in which the carbon molecule has an incomplete negative charge and the metal a fractional positive charge.
Complete answer: The Grignard reaction is an organometallic synthetic reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) is added to a carbonyl gathering in an aldehyde or ketone. This reaction is significant for the development of carbon–carbon bonds. The reaction of a natural halide with magnesium isn't a Grignard reaction, however it gives a Grignard reagent.

When ethylmagnesium bromide reacts with acetonitrile in the presence of oxonium it gives butanone.
Magnesium atom is used in the Grignard reagent because magnesium metal is fairly easily oxidized and the Grignard reagent, while water sensitive, is not so air sensitive, and magnesium metal is cheap, and easy to deploy.
Additional information:
Grignard reagents are made through the expansion of magnesium metal to alkyl or alkenyl halides. The halide can be $Cl,$$Br$,$I,$ (not $F$) . It's marginally simpler to make Grignards from the iodides and bromides, nonetheless. The magnesium is embedding itself between the carbon and the halide. This halides the $X$ alluded to when we allude to Grignard reagents as $RMgX$.They're very acceptable nucleophiles, responding with electrophiles like carbonyl mixtures and epoxides. They're additionally solid bases and will respond with acidic hydrogens.
Note:
Grignard reagents are utilized artificially to frame new carbon–carbon bonds. A Grignard reagent has a polar carbon–magnesium bond in which the carbon molecule has an incomplete negative charge and the metal a fractional positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




