
Complete the below reaction:
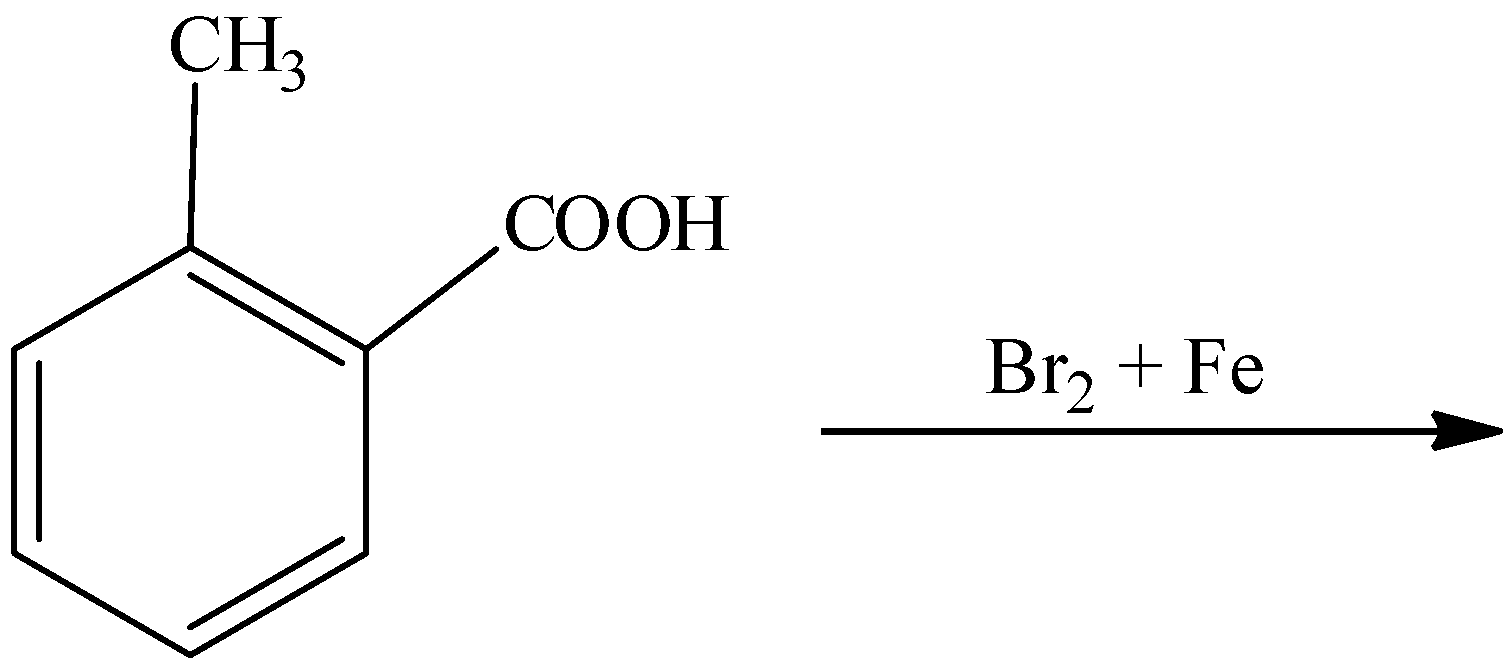

Answer
516k+ views
Hint: We have to know ortho toluic acid is a carboxylic acid which is aromatic in nature. We can write the chemical formula of o-toluic acid as $\left( {C{H_3}} \right){C_6}{H_4}COOH$. We have to know that an isomer of m-toluic acid and p-toluic acid is o-toluic acid. The empirical formula of o-toluic acid is ${C_8}{H_8}{O_2}$.
Complete answer:
We can give IUPAC the name of o-toluic acid as 2-methylbenzoic acid.
We can know that that monosubstituted compound is treated with an electrophile, it will go through electrophilic aromatic replacement reaction and gives a disubstituted compound which might be perceived utilizing the descriptors ortho, meta, and para. On the off chance that the general yield of the ortho product and the para product are higher when compared to that of the meta product, at that point the substituent inside the monosubstituted ring is named an ortho, para coordinating group.
We have to know if the substituents in the aromatic ring deal with the basic reaction or have a direct impact on the shaped positional isomer. In an electrophilic replacement reaction electron giving group normally goes about as directors of ortho/para while electron donating groups goes about as meta directors. The halogen goes about as directors of ortho/para since they require solitary sets of electrons to impart to the aromatic ring.
We have to that methyl group $\left( { - C{H_3}} \right)$ is group which is ortho and para directing whereas carboxyl group $\left( { - COOH} \right)$ is a group that is meta directing. So, we have to know that when a new electrophile enters, it could occupy the position that will be met directly with respect to the carboxyl group and para directing with respect to the methyl group.
We can write the complete reaction as,
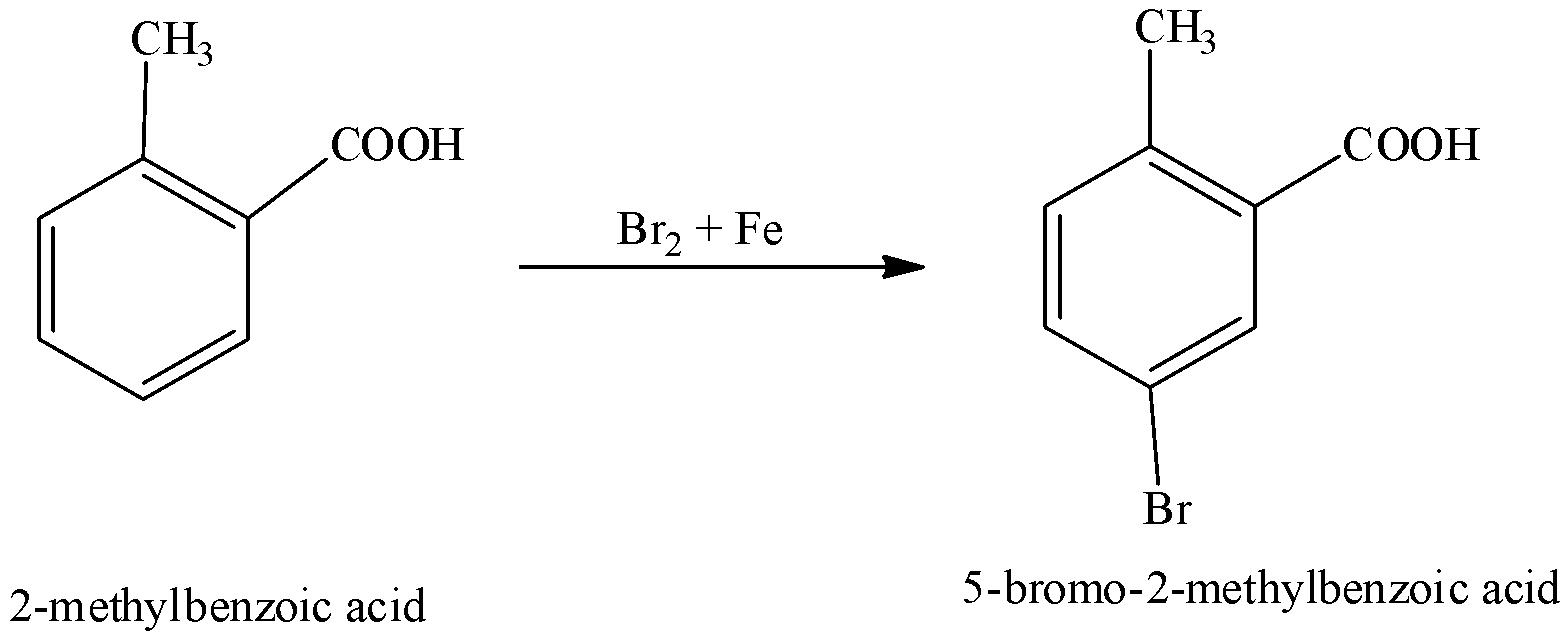
Note:
We have to know that some of the examples of ortho-para directors are hydroxyl groups, alkyl groups, amines, ethers, halogens and thiols. We have to know that some of the examples of meta directors are cyanides, aldehydes, ketones, esters, nitriles, nitro group, sulfones, alkyl ammoniums.
Complete answer:
We can give IUPAC the name of o-toluic acid as 2-methylbenzoic acid.
We can know that that monosubstituted compound is treated with an electrophile, it will go through electrophilic aromatic replacement reaction and gives a disubstituted compound which might be perceived utilizing the descriptors ortho, meta, and para. On the off chance that the general yield of the ortho product and the para product are higher when compared to that of the meta product, at that point the substituent inside the monosubstituted ring is named an ortho, para coordinating group.
We have to know if the substituents in the aromatic ring deal with the basic reaction or have a direct impact on the shaped positional isomer. In an electrophilic replacement reaction electron giving group normally goes about as directors of ortho/para while electron donating groups goes about as meta directors. The halogen goes about as directors of ortho/para since they require solitary sets of electrons to impart to the aromatic ring.
We have to that methyl group $\left( { - C{H_3}} \right)$ is group which is ortho and para directing whereas carboxyl group $\left( { - COOH} \right)$ is a group that is meta directing. So, we have to know that when a new electrophile enters, it could occupy the position that will be met directly with respect to the carboxyl group and para directing with respect to the methyl group.
We can write the complete reaction as,

Note:
We have to know that some of the examples of ortho-para directors are hydroxyl groups, alkyl groups, amines, ethers, halogens and thiols. We have to know that some of the examples of meta directors are cyanides, aldehydes, ketones, esters, nitriles, nitro group, sulfones, alkyl ammoniums.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




