
Complete and rewrite the balanced chemical equation
$Chlorobenzene\xrightarrow[473K,pressure]{NaCN+\,CuCN}\,?$
Answer
531.9k+ views
Hint: In this question, we have to complete the reaction. In this chemical equation, An alkyl halide is reacting in the presence of cyanide, so It is confirmed that alkyl cyanide will be formed as a product from it. Now we can complete this reaction easily.
Complete answer:
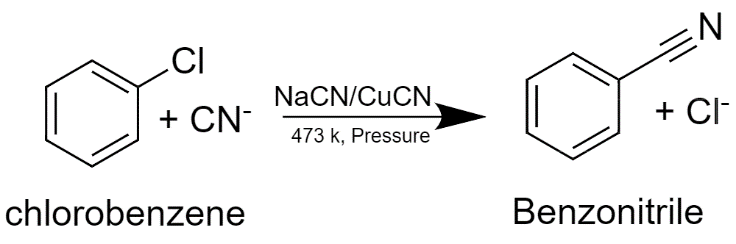
The aromatic organic compound which has a chemical formula (${{C}_{6}}{{H}_{5}}Cl$) is known as Chlorobenzene. It is a common solvent that is a flammable and colorless liquid and is used for manufacturing other chemicals. It is prepared by chlorination of benzene in the presence of a catalyst such as $FeC{{l}_{3}}$ anhydrous aluminum chloride. It is now used for pesticide formation, to remove the grease from automobiles but in the past, it was used to prepare DDT and phenol.
When chlorobenzene is treated with alcoholic sodium cyanide and copper cyanide, then Benzonitrile is formed.
We take cyanide ion because cyanide ion is more nucleophilic if we compare it to the chloride ion. This reaction is known as the Rosenmund Von Braun reaction.
Additional information:
The chemical equation should be balanced because the number of atoms on both sides of the equation should be equal as it sticks to the Law of conservation.
Note:
The aromatic organic compound which has chemical formula (${{C}_{6}}{{H}_{5}}CN$) is known as benzonitrile. It can be written as PhCN. It is a colorless liquid that has a sweet-bitter almond-like odor. It forms coordination compounds with transition metals.
Complete answer:
The aromatic organic compound which has a chemical formula (${{C}_{6}}{{H}_{5}}Cl$) is known as Chlorobenzene. It is a common solvent that is a flammable and colorless liquid and is used for manufacturing other chemicals. It is prepared by chlorination of benzene in the presence of a catalyst such as $FeC{{l}_{3}}$ anhydrous aluminum chloride. It is now used for pesticide formation, to remove the grease from automobiles but in the past, it was used to prepare DDT and phenol.
When chlorobenzene is treated with alcoholic sodium cyanide and copper cyanide, then Benzonitrile is formed.
We take cyanide ion because cyanide ion is more nucleophilic if we compare it to the chloride ion. This reaction is known as the Rosenmund Von Braun reaction.

Additional information:
The chemical equation should be balanced because the number of atoms on both sides of the equation should be equal as it sticks to the Law of conservation.
Note:
The aromatic organic compound which has chemical formula (${{C}_{6}}{{H}_{5}}CN$) is known as benzonitrile. It can be written as PhCN. It is a colorless liquid that has a sweet-bitter almond-like odor. It forms coordination compounds with transition metals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




