
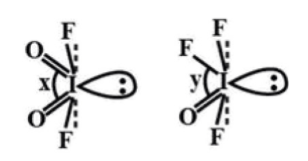
Compare x and y bond angles for the above given molecules.
A. x > y
B. y > x
C. x=y
D. None of these

Answer
578.1k+ views
Hint: Observe the bonding atoms of bond angles x and y. The fluorine atom is more electronegative than the oxygen atom. Determine the type of repulsion present in the given molecules. Lower bond pair-bond pair repulsion lower is the bond angle.
Complete step by step answer:
The structures of molecules given to us are:
In the first structure bond angle \[{\text{O = I = O}}\] is represented by ‘x’. In the second structure bond angle \[{\text{F - I = O}}\] is represented by ‘y’. So in these two structures, there is a difference of one bond. So we can determine the difference in bond angle by comparing the electronegativity of the F and O atom. As the F atom is more electronegative than the O atom it will attract the bond pair of electrons towards itself. So in the second structure bond pair-bond pair repulsion \[{\text{F - I}}\] and \[{\text{I = O}}\] is less compare to bond pair-bond pair repulsion of two \[{\text{I = O}}\] bonds in the first structure.
The bond angle of a molecule having greater bond pair-bond pair repulsion is greater.
So, angle x>y.
Thus, the correct option is (A) x>y
Additional information:
The reported bond angle\[{\text{O = I = O}}\] is \[{\text{100}}^\circ \] and reported bond angle of \[{\text{F - I = O}}\] is \[98^\circ \].
The bond angle depends on the following 3 types of repulsions.
1. Lone pair-lone pair repulsion
2. Bond pair-bond pair repulsion
3. Lone pair-bond pair repulsion
The strongest among all these three repulsions is Lone pair-lone pair repulsion.
Note:
Bond angle of two bonds is based on bonding atoms, lone pair on bonding atoms, type of bonds and electronegativity of bonding atoms. As electronegativity increases from left to right across the period so F atom is more electronegative than O atom. The greater the repulsion of bonding atoms greater is the bond angle.
Complete step by step answer:
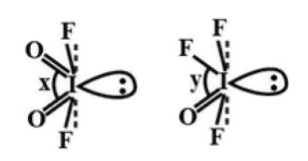
The structures of molecules given to us are:

In the first structure bond angle \[{\text{O = I = O}}\] is represented by ‘x’. In the second structure bond angle \[{\text{F - I = O}}\] is represented by ‘y’. So in these two structures, there is a difference of one bond. So we can determine the difference in bond angle by comparing the electronegativity of the F and O atom. As the F atom is more electronegative than the O atom it will attract the bond pair of electrons towards itself. So in the second structure bond pair-bond pair repulsion \[{\text{F - I}}\] and \[{\text{I = O}}\] is less compare to bond pair-bond pair repulsion of two \[{\text{I = O}}\] bonds in the first structure.
The bond angle of a molecule having greater bond pair-bond pair repulsion is greater.
So, angle x>y.
Thus, the correct option is (A) x>y
Additional information:
The reported bond angle\[{\text{O = I = O}}\] is \[{\text{100}}^\circ \] and reported bond angle of \[{\text{F - I = O}}\] is \[98^\circ \].
The bond angle depends on the following 3 types of repulsions.
1. Lone pair-lone pair repulsion
2. Bond pair-bond pair repulsion
3. Lone pair-bond pair repulsion
The strongest among all these three repulsions is Lone pair-lone pair repulsion.
Note:
Bond angle of two bonds is based on bonding atoms, lone pair on bonding atoms, type of bonds and electronegativity of bonding atoms. As electronegativity increases from left to right across the period so F atom is more electronegative than O atom. The greater the repulsion of bonding atoms greater is the bond angle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




