
Compare x and y bond angle from the given molecules.
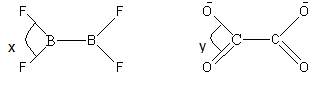
(A) x > y
(B) y > x
(C) x = y
(D) None of these
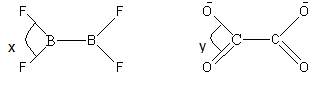
Answer
594k+ views
Hint:
The bond angles between two bonds are decided by factors like bond pair-bond pair, bond pair-lone pair and lone pair-lone pair repulsions. Lone pair-lone pair repulsion is the highest amongst all the repulsions.
Complete answer:
We know that Boron in the above structure has one lone pair and Fluorine has three lone pairs. Oxygen will be having two lone pairs normally but if it is negatively charged then, it will have three line pairs like Fluorine. The structure will be like,
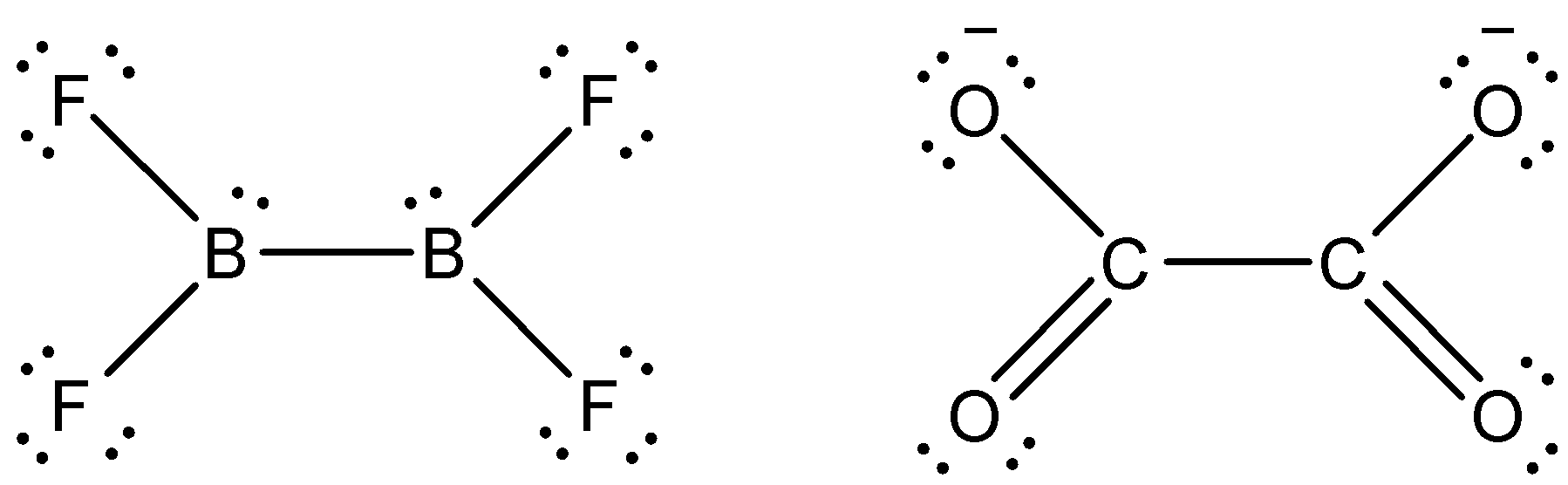
We can see that the x and y bond angle shows F-B-F and O-C-O bond angles respectively.
We can see that two fluorine atoms are adjacent to each other and will feel lone pair- lone pair repulsion, but the same case is here in the other example where one oxygen atom is having three lone pairs and another is having two lone pairs. Remember that only one lone pair-lone pair repulsion is possible here as the geometry of the compound will not allow other lone pairs to repel each other. So, lone pair- lone pair repulsion is relatively the same in both the compounds.
- Now in the other compound, there is Carbon-carbon double bond, which involves overlapping of orbitals from side and hence electron density will be there in larger areas. That bond pair will create extra repulsion in comparison to the boron containing compound.
So, we can say that with an extra repulsive force between oxygen lone pairs and bond pairs of carbon-carbon double bond, bond angle y will be greater than x.
So, correct answer is (B) y > x
Additional Information:
- Highest repulsion is of lone pair-lone pair repulsion.
- The lone pair on boron atoms do not interact with other lone pairs because it is situated at some distance and hence repulsive forces will be very less.
Note:
Remember that as oxygen has less lone pairs, do not directly choose that its compound will have less bond angle, the repulsion between bond pair and lone pair also need to be taken in consideration.
The bond angles between two bonds are decided by factors like bond pair-bond pair, bond pair-lone pair and lone pair-lone pair repulsions. Lone pair-lone pair repulsion is the highest amongst all the repulsions.
Complete answer:
We know that Boron in the above structure has one lone pair and Fluorine has three lone pairs. Oxygen will be having two lone pairs normally but if it is negatively charged then, it will have three line pairs like Fluorine. The structure will be like,

We can see that the x and y bond angle shows F-B-F and O-C-O bond angles respectively.
We can see that two fluorine atoms are adjacent to each other and will feel lone pair- lone pair repulsion, but the same case is here in the other example where one oxygen atom is having three lone pairs and another is having two lone pairs. Remember that only one lone pair-lone pair repulsion is possible here as the geometry of the compound will not allow other lone pairs to repel each other. So, lone pair- lone pair repulsion is relatively the same in both the compounds.
- Now in the other compound, there is Carbon-carbon double bond, which involves overlapping of orbitals from side and hence electron density will be there in larger areas. That bond pair will create extra repulsion in comparison to the boron containing compound.
So, we can say that with an extra repulsive force between oxygen lone pairs and bond pairs of carbon-carbon double bond, bond angle y will be greater than x.
So, correct answer is (B) y > x
Additional Information:
- Highest repulsion is of lone pair-lone pair repulsion.
- The lone pair on boron atoms do not interact with other lone pairs because it is situated at some distance and hence repulsive forces will be very less.
Note:
Remember that as oxygen has less lone pairs, do not directly choose that its compound will have less bond angle, the repulsion between bond pair and lone pair also need to be taken in consideration.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




