
Compare the structures of \[S{O_3}\] to \[P{F_3}\] and explain why they have different molecular shapes?
Answer
501.6k+ views
Hint: We need to know that the atomic number of oxygen is \[8\]. The atomic number of fluorine is \[9\]. The mass number of oxygen is \[16\]. The mass number of fluorine is \[18.99\]. The symbol of fluorine is \[{\text{F}}\]. Fluorine is one of the halogens in the periodic table. The symbol of oxygen is \[O\]. The atomic number of sulphur is \[16\]. The atomic number of phosphorus is \[15\]. The mass number of sulphur is \[32\]. The mass number of phosphorus is \[30\]. The symbol of sulphur is \[S\]. The symbol of phosphorus is \[P\].
Complete answer:
The molecular formula of sulphur trioxide is \[S{O_3}\].
The molecular formula of phosphorus trifluoride is \[P{F_3}\].
The structure of sulphur trioxide is given below,
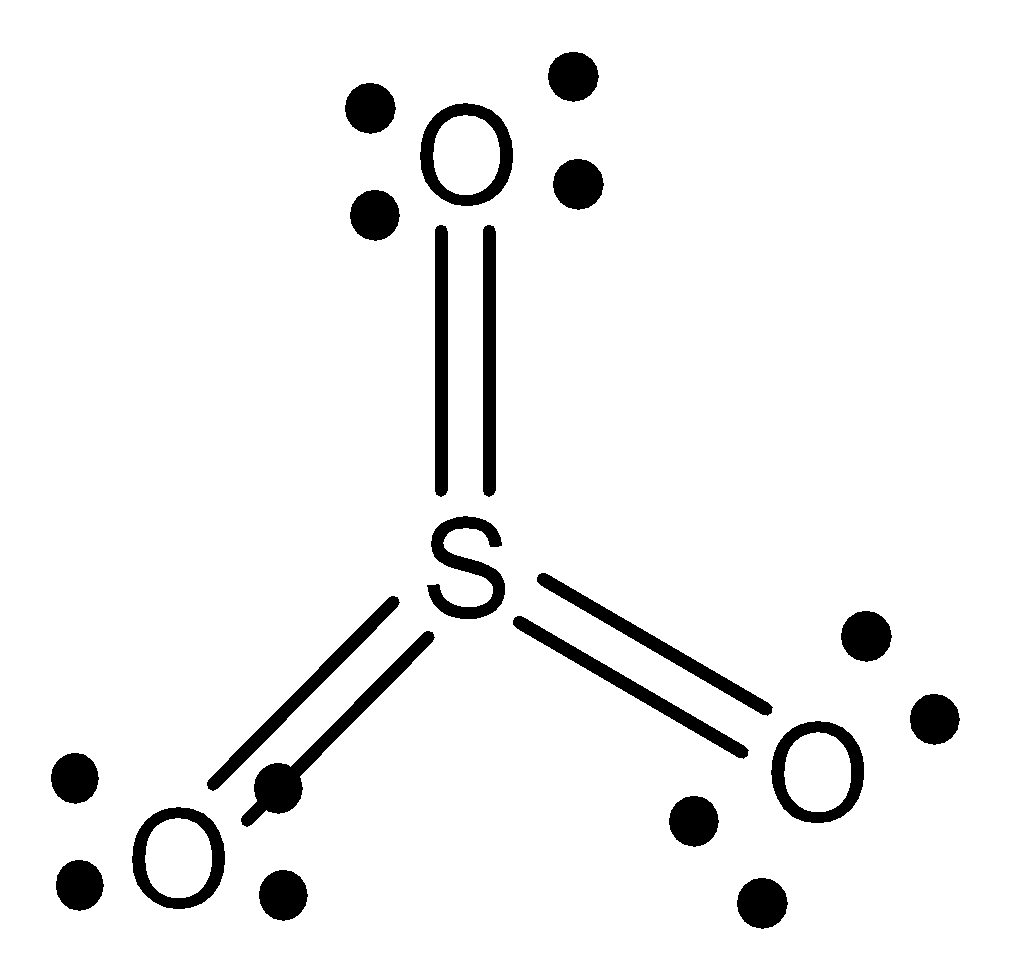
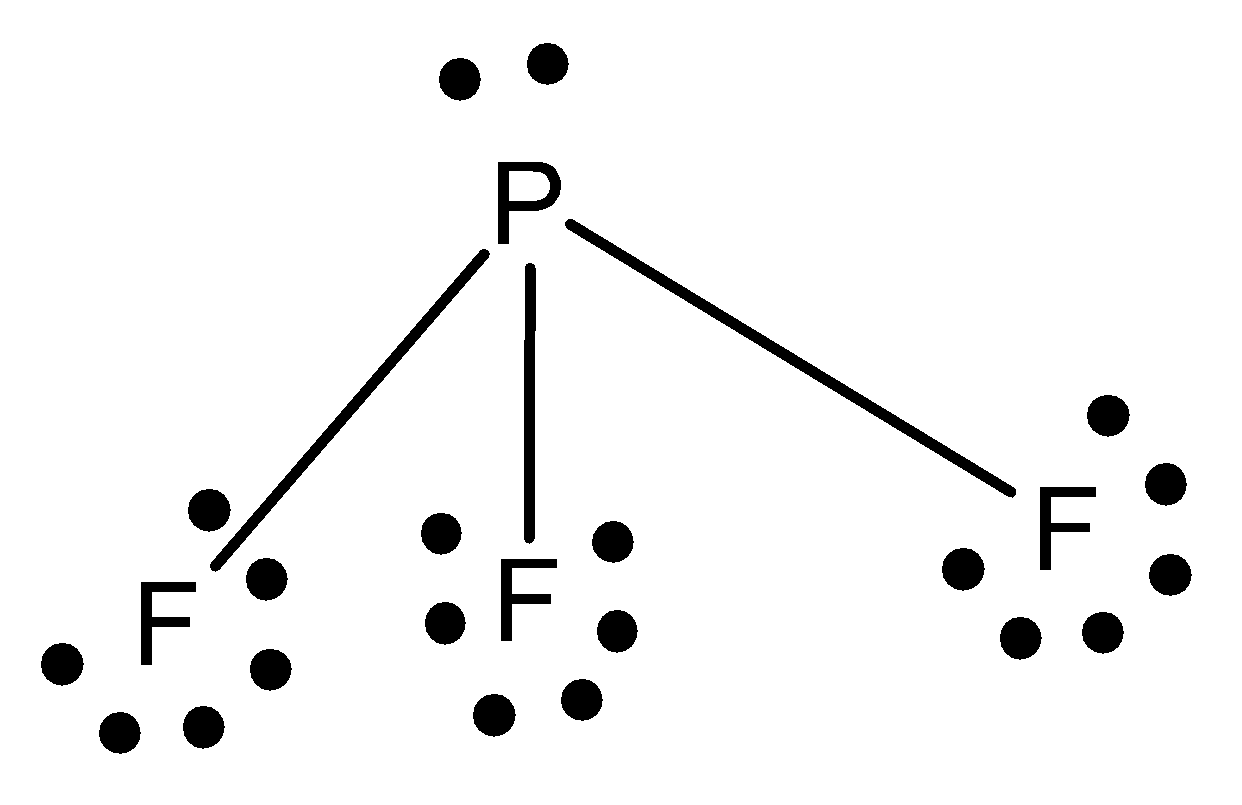
The structure of sulphur phosphorus trifluoride is given below,
The hybridisation of sulphur trioxide is \[s{p^2}\].
The hybridisation of phosphorus trifluoride is \[s{p^3}\].
The geometry of sulphur trioxide is trigonal planar.
The geometry of phosphorus trifluoride is tetrahedral.
The shape of sulphur trioxide is trigonal planar.
The shape of phosphorus trifluoride is pyramidal.
Sulphur trioxide and phosphorus trifluoride have different molecular shapes, because of the geometry and hybridisation of the molecules.
Sulphur trioxide is non-polar, because of the trigonal planar shapes all the polarity of the molecules comes to zero due to all the dipole moment of the molecule is cancel each other and phosphorus trifluoride is polar, because of the pyramidal shape all the polarity of the molecules not comes to zero due to all the dipole moment of the molecule is not cancel each other.
Note:
As we know that in chemistry, periodic tables play a vital role. In the periodic table there are totally \[118\] elements. In the periodic table there are totally \[18\] columns and \[7\] rows. The columns are called groups. Hence, \[18\] groups in the periodic table. The rows are called periods. Hence, totally \[7\] period in the table. The atomic number of the element is nothing but the number of electrons or number of protons. The mass number of the atom is nothing but the sum of the number of protons and number of neutrons.
Complete answer:
The molecular formula of sulphur trioxide is \[S{O_3}\].
The molecular formula of phosphorus trifluoride is \[P{F_3}\].
The structure of sulphur trioxide is given below,

The structure of sulphur phosphorus trifluoride is given below,

The hybridisation of sulphur trioxide is \[s{p^2}\].
The hybridisation of phosphorus trifluoride is \[s{p^3}\].
The geometry of sulphur trioxide is trigonal planar.
The geometry of phosphorus trifluoride is tetrahedral.
The shape of sulphur trioxide is trigonal planar.
The shape of phosphorus trifluoride is pyramidal.
Sulphur trioxide and phosphorus trifluoride have different molecular shapes, because of the geometry and hybridisation of the molecules.
Sulphur trioxide is non-polar, because of the trigonal planar shapes all the polarity of the molecules comes to zero due to all the dipole moment of the molecule is cancel each other and phosphorus trifluoride is polar, because of the pyramidal shape all the polarity of the molecules not comes to zero due to all the dipole moment of the molecule is not cancel each other.
Note:
As we know that in chemistry, periodic tables play a vital role. In the periodic table there are totally \[118\] elements. In the periodic table there are totally \[18\] columns and \[7\] rows. The columns are called groups. Hence, \[18\] groups in the periodic table. The rows are called periods. Hence, totally \[7\] period in the table. The atomic number of the element is nothing but the number of electrons or number of protons. The mass number of the atom is nothing but the sum of the number of protons and number of neutrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




